Translate this page into:
Papillary eccrine adenoma associated with syringocystadenoma papilliferum
2 Department of Pathology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, India
Correspondence Address:
Neha Meena
Department of Dermatology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi - 110 001
India
| How to cite this article: Meena N, Sharma PK, Kumar S, Bhardwaj M. Papillary eccrine adenoma associated with syringocystadenoma papilliferum. Indian J Dermatol Venereol Leprol 2018;84:322-324 |
Sir,
Papillary eccrine adenoma, first described by Rulon and Helwig in 1977, is an uncommon, longstanding, benign tumor of sweat glands, usually located on the extremities of darker skin individuals, especially females.[1] Clinically, it presents as an asymptomatic, occasionally painful or pruritic, solitary, firm dermal nodule, though other clinical variants have been reported.[1],[2] Although malignant transformation of papillary eccrine adenoma is very rare, complete excision of the tumor or Mohs micrographic surgery is preferred.[2],[3] We report a case of papillary eccrine adenoma with syringocystadenoma papilliferum, for its rare presentation.
A 15-year-old boy presented with asymptomatic, pinkish, raised lesions on back of 5-years duration. The lesions slowly progressed to the present size. On examination, multiple grouped pink to violaceous, dome-shaped, coalescing papules of size 0.5 cm × 0.5 cm, with perilesional hyperpigmentation, were present on the left lateral aspect of lower back in the paravertebral region. A few papules showed central umbilication, while others showed erosions [Figure - 1]. Punch biopsy from a papule revealed thick stratum corneum, acanthosis and mild spongiosis with dense band-like inflammatory infiltrate composed of lymphocytes and histiocytes in upper dermis. Mild dermis showed variable-sized dilated tubular structures lined by two layers of cuboidal epithelium with stratification at places. The lumen contained amorphous and granular eosinophilic secretions. Intervening stroma showed fibrovascular tissue. Features were consistent with papillary eccrine adenoma [Figure - 2]a. It showed positivity to S100 and epithelial membrane antigen [Figure - 2]b and [Figure - 2]c. Punch biopsy from another papule showed orthokeratosis, acanthosis and downward cystic invagination from the epidermis. The upper portion of the invagination was lined by keratinized squamous cells and lower portion showed papillary projection into the cystic cavity, lined by two rows of cells. Luminal row of cells was composed of high columnar cells with oval nuclei and faintly eosinophilic cytoplasm; outer row of cells was consisting of small cuboidal cells with round nuclei and scant cytoplasm. At some places, luminal layer was arranged in multiple layers. Dermis showed lymphoplasmacytic inflammatory infiltrate. Features were consistent with syringocystadenoma papilliferum [Figure - 3]a and [Figure - 3]b.
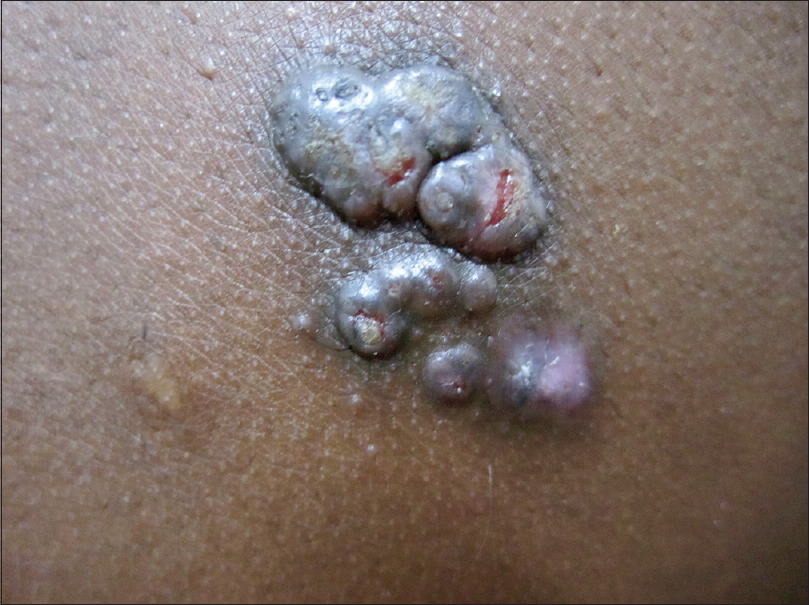 |
| Figure 1: Multiple, grouped, pink to violaceous, dome-shaped papule on back |
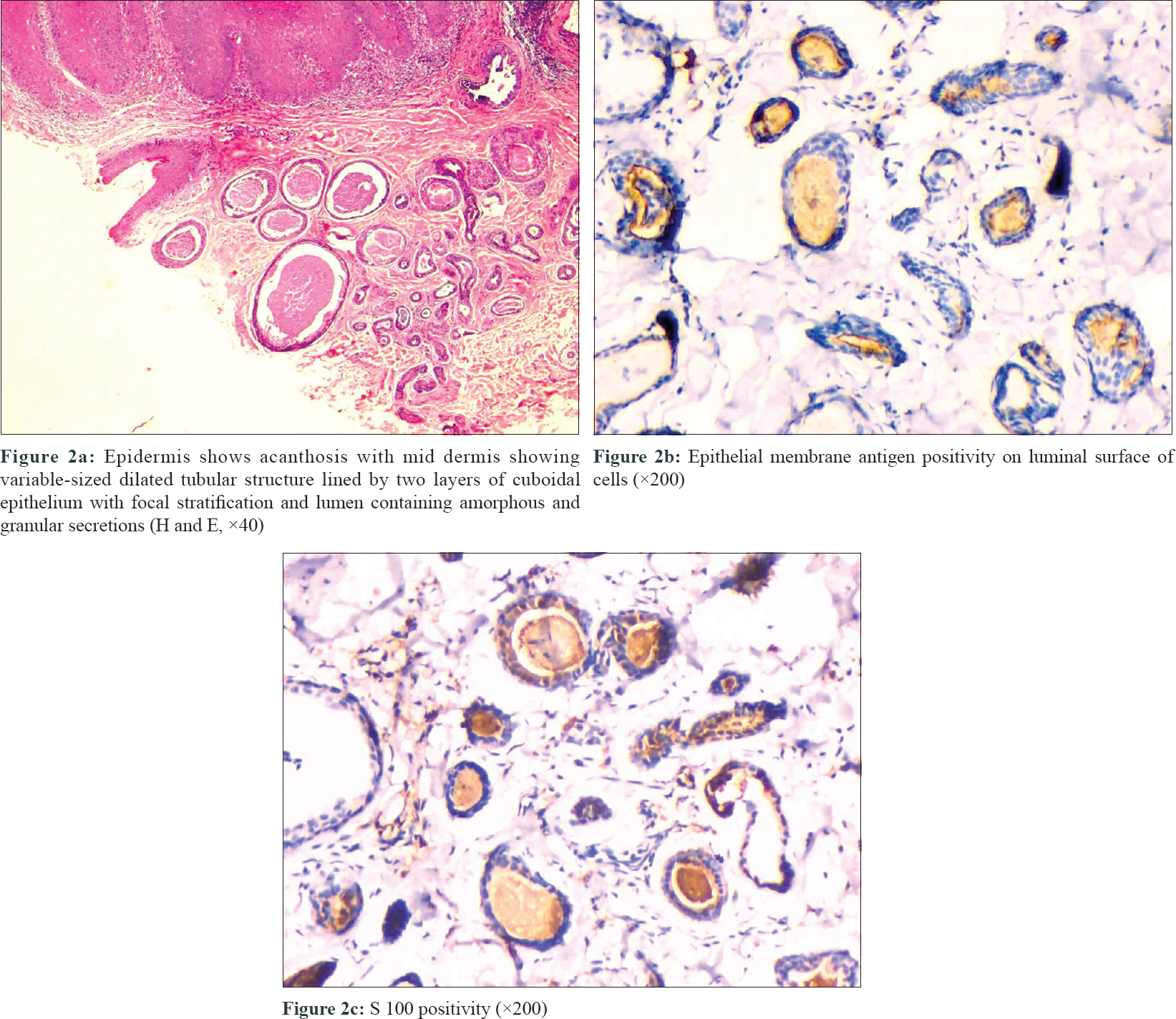 |
| Figure 2 |
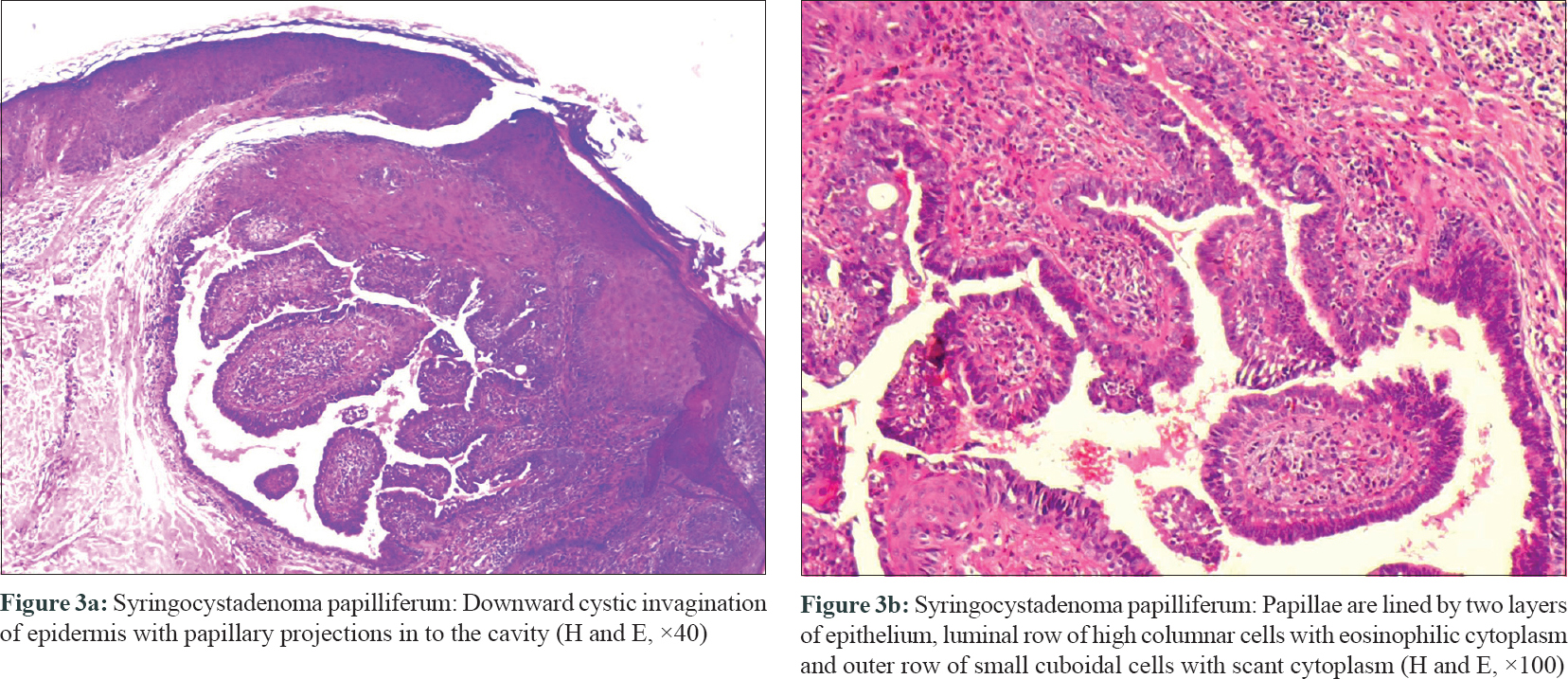 |
| Figure 3 |
Syringocystadenoma papilliferum is a benign hamartomatous adnexal tumor, which presents either as solitary, multiple or linear verrucous, dome-shaped, translucent or pinkish, grouped papules or plaques.[4] The origin of syringocystadenoma papilliferum is still controversial. Presumably, it arises from pluripotent cells with the potential to exhibit either apocrine or eccrine lineage of which apocrine differentiation is more common.[4] Syringocystadenoma papilliferum can be easily differentiated from papillary eccrine adenoma by its characteristic histology.[4],[5] Syringocystadenoma papilliferum shows multiple epithelial invaginations extending down from the epidermis and contains numerous papillae which consist of a dermal fibrovascular core, lined by two layers of epithelial cells, luminal columnar cells with decapitation secretion and outer cuboidal cells. A characteristically marked plasmacytic inflammatory cell infiltrate is present within the fibrous cores.[4],[5] Similar to our case, Coyne and Fitzgibbon also reported this rare association of syringocystadenoma papilliferum and papillary eccrine adenoma in a 67-year-old man with a scrotal condyloma.[6]
The term “Tubulopapillary hidradenoma” was introduced to describe benign sweat gland tumors, characterized by combining ductal as well as apocrine and eccrine glandular differentiation which include both papillary eccrine adenoma and tubular apocrine adenoma.[4] Tubular apocrine adenoma usually presents on the scalp as superficial dermal nodule and shares the histological features of papillary eccrine adenoma, except that the latter has a peripheral myoepithelial cell layer. Intraluminal papillary projections and eosinophilic amorphous material are also found in tubular apocrine adenoma. However, immunohistochemical positivity with S100, carcinoembryonic antigen and epithelial membrane antigen suggests eccrine origin of papillary eccrine adenoma.[4],[5] Eccrine differentiation of papillary eccrine adenoma is further suggested by positive amylophosphorylase reaction, while the lack of staining with acid phosphate rules out apocrine differentiation.[4] Decapitation secretion is not found in eccrine adenoma as compared to apocrine adenoma.
Differential diagnosis of papillary eccrine adenoma includes digital papillary adenocarcinoma. Clinically, digital papillary adenocarcinoma presents as a rapidly progressing nodule with acral distribution in elderly individuals with infiltration in surrounding skin and high chance of recurrence.[4],[5] Histologically, it shows both nodular solid and cystic granular pattern of cuboidal or low-columnar epithelial cells, with back-to-back arrangement or cribriform pattern of glands.[4],[5] Multiple cystic spaces, containing eosinophilic material, with luminal papillary projections are characteristically present. Decapitation secretion, cytonuclear atypia, increased mitotic activity or tumor necrosis may also be seen, albeit very uncommon. Another differential is syringocystadenocarcinoma papilliferum, characterized histologically by disorderly arrangement of papillary projections, cytological atypia and loss of double-layered epithelium.[5]
The reports of coexistence of syringocystadenoma papilliferum with tubular apocrine adenoma and/or with papillary eccrine adenoma suggest that tubular apocrine adenoma, papillary eccrine adenoma and syringocystadenoma papilliferum may represent a spectrum of inter-related tumors.[5],[6],[7],[8] Our case adds to the evidence of this spectrum of differentiation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Rulon DB, Helwig EB. Papillary eccrine adenoma. Arch Dermatol 1977;113:596-8.
[Google Scholar]
|
| 2. |
Laxmisha C, Thappa DM, Jayanthi S. Papillary eccrine adenoma. Indian J Dermatol Venereol Leprol 2004;70:370-2.
[Google Scholar]
|
| 3. |
Jung JH, Im S, Kang SJ, Kim GM, Han KT, Yoo JY, et al. A cutaneous myoepithelial carcinoma arising in a papillary eccrine adenoma. Korean J Pathol 2011;45:644-9.
[Google Scholar]
|
| 4. |
Ahmed TS, Priore JD, Seykora JT. Tumors of the epidermal appendages. In: Elder D, Elenitsas R, Johnson BL Jr., Murphy GF, Xu X, editors. Lever's Histopathology of the Skin. 10th ed. New Delhi: Lippincott Williams and Wilkins Publication; 2009. p. 851-910.
[Google Scholar]
|
| 5. |
Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms – Part 2: An approach to tumours of cutaneous sweat glands. J Clin Pathol 2007;60:145-59.
[Google Scholar]
|
| 6. |
Coyne JD, Fitzgibbon JF. Mixed syringocystadenoma papilliferum and papillary eccrine adenoma occurring in a scrotal condyloma. J Cutan Pathol 2000;27:199-201.
[Google Scholar]
|
| 7. |
Hsu PJ, Liu CH, Huang CJ. Mixed tubulopapillary hidradenoma and syringocystadenoma papilliferum occurring as a verrucous tumor. J Cutan Pathol 2003;30:206-10.
[Google Scholar]
|
| 8. |
Ishiko A, Shimizu H, Inamoto N, Nakmura K. Is tubular apocrine adenoma a distinct clinical entity? Am J Dermatopathol 1993;15:482-7.
[Google Scholar]
|
Fulltext Views
4,067
PDF downloads
2,523





