Translate this page into:
Parthenium dermatitis treated with azathioprine weekly pulse doses
Correspondence Address:
Kaushal K Verma
Department of Dermatology and Venereology,All India Institute of Medical Sciences, New Delhi 110029
India
| How to cite this article: Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol 2006;72:24-27 |
Abstract
Background: Parthenium dermatitis is a serious problem in India. Corticosteroids are the mainstay of treatment but the prolonged use of corticosteroids can cause serious side effects. Azathioprine used in daily doses has been shown to be effective. Aim: We have evaluated the effectiveness of azathioprine weekly pulse doses for the treatment of parthenium dermatitis. Methods: Twelve patients, ten males and two females, aged between 39 and 65 years (mean � SD = 53.5 � 8.7) having air-borne contact dermatitis to Parthenium hysterophorus for 3-19 years (mean = 6.33) were included in the study. The diagnosis in each patient was confirmed by patch-testing. The severity of the disease was determined by clinical severity score (CSS) on the basis of erythema, itching, type of lesions, and areas of body involved. Results: The pretreatment CSS in these patients varied from 29.7 to 55.5 (mean � SD: 40.40 � 7.95). After clinical and laboratory evaluation, the patients were treated with 300-mg azathioprine once-weekly doses for 6 months. Clinical and laboratory evaluations were repeated at weeks 1, 2, and then every 4 weeks until the end of therapy to evaluate the therapeutic response and side effects. The response was excellent (80-100% clearance of disease) in seven (58.33%) patients and good (60% clearance) in five (41.66%) patients. The post-treatment CSS decreased from the mean � SD of 40.4 � 7.95 to 10.9 � 8.43 (P = 0.002). There were no significant side effects of the therapy. Conclusions: In this preliminary open study, azathioprine in weekly pulse doses has been found to be effective without any serious adverse effects in the treatment of parthenium dermatitis. The cost of therapy with this regimen is reduced by 60%.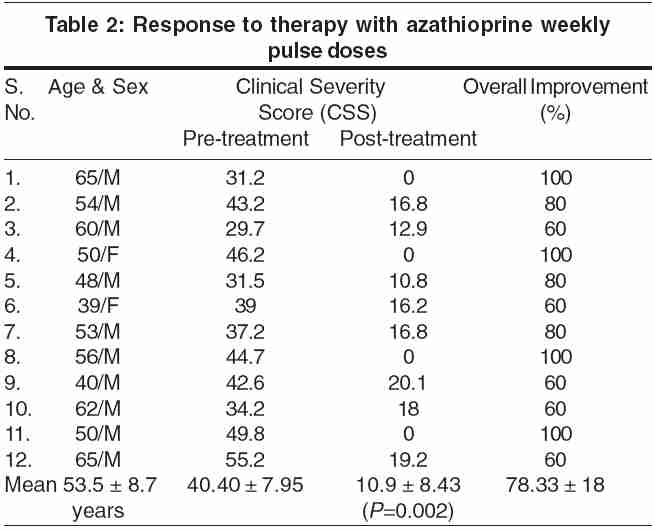



INTRODUCTION
Parthenium dermatitis is the commonest type of air-borne contact dermatitis in India. It is caused by the compositae weed Parthenium hysterophorus . The disease occurs in sensitized individuals and usually manifests as itchy, erythematous papules and plaques on exposed areas of the body, such as the face (including the upper eyelids), neck, "V" area of the upper chest, flexures of the forearms, and the antecubital and popliteal fossae.[1],[2] The type of lesions and pattern of dermatitis may vary in some individuals.[3]
P. hysterophorus grows profusely during the rainy season almost throughout the country. However, the plant is present perennially and so is the antigen in the atmosphere; therefore the patients suffer from dermatitis throughout the year with exacerbation during rainy seasons. Parthenium dermatitis is a very distressing and intractable problem in India. Attempts to eradicate the weed have been unsuccessful. Efforts to desensitize the patients with plant material have given inconsistent and unreliable results.[4] Corticosteroids, therefore, have been the mainstay of treatment. However, the long-term use of corticosteroids has often caused serious and sometimes irreversible side effects.[5],[6] In our previous studies, we have been able to achieve remissions in patients of parthenium dermatitis with azathioprine, used as daily doses as well as monthly bolus doses, with negligible side effects. [7],[8],[9] In this study we evaluated the therapeutic response and side effects of azathioprine pulse, 300 mg, given once weekly in patients of parthenium dermatitis.
METHODS
Twelve patients with a clinical diagnosis of air-borne contact dermatitis by P. hysterophorus were included in the study. The patients were patch-tested with aqueous extract of Parthenium and the titer of contact hypersensitivity (TCH) was determined by the technique standardized by Pasricha et al .[10],[11] Patients with active liver disease, renal or hematological disorders, and pregnant and lactating females were excluded from the study.
A detailed clinical examination was undertaken after written consent. The severity of the disease was assessed by determining the clinical severity score (CSS), a scoring system developed by us for determining the severity of the dermatitis in parthenium dermatitis based on erythema, itching, type of lesions, and area of involvement. To determine the CSS, (a) erythema and (b) itching were graded as mild, moderate, and severe, (c) type(s) of lesions as papules, plaques, lichenified papules, and plaques and exudative lesions, and (d) areas of the body involved were assessed. Each of these parameters was assigned a number (score) depending on the severity of the symptom or the approximate area of the body involved (score of 1 was given to an approximate area of 9% involvement). The details appear in [Table - 1]. The CSS was calculated by adding (a), (b), (c), and (d) and multiplying it by 3 to get a maximum score of 99.
Baseline investigations, consisting of complete blood count, hepatic and renal function tests, serum electrolytes, routine and microscopic examination of urine and stool, chest X-ray, and electrocardiography, were done. Estimation of thiopurine methyl transferase (TPMT) activity could not be done in these patients because this facility was not available.
Patients with a positive patch test to P. hysterophorus and normal investigations were given one tablet of azathioprine (50 mg) as a test dose and observed for a period of 48 hours. Thereafter, they were given azathioprine 300 mg (six tablets of 50 mg each, together) orally after a meal once every week for 6 months. Patients already on some form of treatment were given a wash-off period of 2 weeks before starting the therapy; 10-mg cetirizine hydrochloride orally once daily and clobetasol propionate cream (0.05%) applied twice daily was also given to patients for symptomatic relief.
Clinical and laboratory evaluation (complete blood counts and hepatic and renal function tests) were repeated at 1, 2, and 4 weeks after the initiation of therapy to determine any side effects. Thereafter, clinical and laboratory evaluations were done at 4-week intervals to assess the therapeutic response and side effects. The therapeutic response was determined by assessing the overall post-treatment improvement in dermatitis and itching. It was considered to be excellent if the improvement was more than 75%, good if it was 50-75%, and poor if the improvement was < 50%. The post-treatment CSS was also determined to evaluate the response to therapy.
RESULTS
Of the 12 patients included, there were 10 males and 2 females, between 39 and 65 years (mean ± SD = 53.5 ± 8.7 years) of age having dermatitis on face, neck, upper chest, dorsae of hands and feet since 3-19 years (mean 6.33 years). The pretreatment CSS varied from 29.7 to 55.5 (mean ± SD: 40.40 ± 7.95). The pretreatment TCH values ranged from undiluted positive to 1:1000. All the 12 patients received the treatment for 6 months.
At the end of 6 months of therapy, there was an excellent response in seven (58.33%) patients and a good response in five (41.66%). No patient had a poor or no response. The post-treatment CSS became zero in all four patients who had 100% improvement, whereas it reduced significantly in all other patients with substantial reduction of CSS (from a pretreatment mean ± SD of 40.40 ± 7.95 to a post-treatment mean ± SD CSS of 10.9 ± 8.43; P = 0.002] [Table - 2]. Concomitant oral betamethasone (2 mg daily) had to be given to two patients with severe disease for 4 weeks in the initial phase of the therapy, whereas in one patient with thick lichenified plaques it had to be used for 16 weeks to control the disease. The post-treatment patch test became negative in two patients, decreased by one dilution in two patients, increased by one dilution in one patient, and remained unchanged in the remaining patients.
Most patients tolerated the therapy well but two patients had vomiting after the initial doses of azathioprine; this was managed by administration of the antiemetic domperidone (10 mg orally) half an hour before the azathioprine dose. In these patients, while on therapy, the vomiting subsided after two and four doses of the drug, respectively, and they continued the therapy further without antiemetics. Two patients complained of anorexia and experienced weight loss of 2 and 3 kg each, whereas one patient had paresthesia of the hands. The treatment did not have to be discontinued owing to side effects in any of these patients. The laboratory parameters also remained within the normal range in all these patients while on treatment.
DISCUSSION
Parthenium dermatitis is a T-cell-mediated immune injury to the skin caused by activated lymphocytes in P. hysterophorus -sensitized individuals. Because the plant grows perennially almost throughout the country, these patients require regular treatment almost throughout the year. Long-term use of systemic corticosteroids induces several serious, and sometimes irreversible, side effects.[5],[6] Hence, azathioprine has been used as a corticosteroid-sparing agent in these patients.[7],[8] Azathioprine is a 6-mercaptopurine derivative, which inhibits purine synthesis and acts as a potent immunosuppressive and a powerful anti-inflammatory agent.[12],[13] Its immunosuppressive effect is owing to inhibition of the activated T-lymphocytes,[14] the cells primarily responsible for the dermatitis. In our previous studies, we have demonstrated that azathioprine given in a dose of 100 mg daily with or without a 300 mg monthly bolus dose could induce clinical remissions in these patients.[7],[8] The drug has been found to be safe even on long-term use without any significant clinical or biochemical side effects.[9],[15],[16]
In this study all patients responded to azathioprine in a 300 mg bolus dose every week. Seven (58.33%) out of 12 patients had 80-100% improvement whereas five (41.66%) patients had 60% improvement. The response was noticed after 4-6 weeks of therapy. Five of the 12 patients had side effects, which were managed conservatively; in none of the patients, however, did the therapy need to be discontinued. The laboratory parameters also remained within normal limits during the course of therapy. Before starting azathioprine it is recommended that the TPMT activity should be measured in patients if the facilities are available.
The other advantages of this regimen were that the patients had to take only four doses of azathioprine in a month, which improved the compliance, and the cost of therapy was reduced by 60% compared with daily azathioprine therapy. Our study shows that weekly pulse doses of azathioprine are effective and safe in the treatment of parthenium dermatitis. However, larger controlled studies are required before it can be recommended as a substitute for daily treatment with azathioprine or corticosteroids for the treatment of parthenium dermatitis.
| 1. |
Sharma SC, Kaur S. Contact dermatitis from composite plants. Indian J Dermatol Venereol Leprol 1990;56:27-30.
[Google Scholar]
|
| 2. |
Verma KK, Sirka CS, Ramam M. Contact dermatitis due to plants: challenges and prospects. Indian Pract 2001;54:791-6.
[Google Scholar]
|
| 3. |
Verma KK, Sirka CS, Ramam M, Sharma VK. Parthenium dermatitis presenting as photosensitive lichenoid eruption: a new clinical variant. Contact Dermatitis 2002;46:286-9.
[Google Scholar]
|
| 4. |
Srinivas CR, Krupashankar DS, Singh KK. Oral hyposensitization in parthenium dermatitis. Contact Dermatitis 1998;18:242.
[Google Scholar]
|
| 5. |
Gallant C, Kenny P. Oral glucocorticoids and their complications: a review. J Am Acad Dermatol 1968;14:161-77.
[Google Scholar]
|
| 6. |
Storrs FJ. Use and abuse of systemic corticosteroid therapy. J Am Acad Dermatol 1979;1:95-105.
[Google Scholar]
|
| 7. |
Verma KK, Pasricha JS. Azathioprine as a corticosteroid-sparing agent in air-borne contact dermatitis. Indian J Dermatol Venereol Leprol 1996;62:30-2.
[Google Scholar]
|
| 8. |
Verma KK, Manchanda Y, Pasricha JS. Azathioprine as a corticosteroid-sparing agent for the treatment of dermatitis caused by the weed parthenium. Acta Dermatol Venereol 2000;80:31-2.
[Google Scholar]
|
| 9. |
Verma KK, Manchanda Y. Long-term safety and toxicity of azathioprine in patients with air-borne contact dermatitis. Indian J Dermatol Venereol Leprol 2001;67:75-7.
[Google Scholar]
|
| 10. |
Pasricha JS, Singh SN. Evaluation of the antigen-impregnated-discs for patch tests. Indian J Dermatol Venereol Leprol 1982;48:327-9.
[Google Scholar]
|
| 11. |
Pasrichs JS. Titre of contact hypersensitivity (TCH) as a means of determining the degree of hypersensitivity in contact dermatitis. Ind J Dermatol Venereol Leprol 1986; 52: 195-7.
[Google Scholar]
|
| 12. |
Ahmed AR, Mox R. Azathioprine. Int J Dermatol 1981;20:461-7.
[Google Scholar]
|
| 13. |
McDonald GJ. Cytotoxic agents for use in dermatology. J Am Acad Dermatol 1985;12:753-75.
[Google Scholar]
|
| 14. |
Thomas CW, Myhre GM, Tschumper R, Sreekumar R, Jelinek D, Mc Kean DJ. Selective inhibition of inflammatory gene expression in activated T lymphocytes: a mechanism of immune suppression by thiopurines. J Pharmacol Exp Ther 2005;312:537-45.
[Google Scholar]
|
| 15. |
Srinivas CR, Balachandran C, Shenoi SD, Acharya S. Azathioprine in the treatment of parthenium dermatitis. Br J Dermatol 1991;124:394-5.
[Google Scholar]
|
| 16. |
Sharma VK, Chakrabarti A, Mahajan V. Azathioprine in the treatment parthenium dermatitis. Int J Dermatol 1998;37:299-302.
[Google Scholar]
|
Fulltext Views
4,809
PDF downloads
2,963





