Translate this page into:
Pattern of use of biologics in psoriasis among Indian dermatologists – a cross sectional survey
Corresponding author: Dr. Disha Dabbas, Department of Dermatology, Command Hospital, Chandi Mandir, Haryana, India. dishadabbas@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Neema S, Dabbas D, Radhakrishnan S, Yadav AK. Pattern of use of biologics in psoriasis among Indian dermatologists – A cross sectional survey. Indian J Dermatol Venereol Leprol 2022;88:515-8.
Abstract
Background and Aims
Biologics are a relatively new class of highly effective drugs in the management of psoriasis. They act on specific immune processes, achieve rapid and sustained clearance and do not cause target organ damage unlike conventional systemic therapy. It appears that their use in our country is not as widespread as in developed nations despite these benefits ; their prohibitive cost may be a major factor for the limited usage. This survey aimed to find out the extent of use and factors hindering usage of biologics for the management of psoriasis by Indian dermatologists.
Methods
It was a cross-sectional questionnaire based study. The questionnaire was designed after a focussed group discussion, followed by validation. The survey was sent in the form of a link to Indian dermatologists. The responses were recorded in excel-sheet and the data was analyzed by SPSS ver 25.
Results
Of the 310 participants who took part, 287 completed the survey. Two hundred (70%) were users of biologics, while 87 (30%) had never used them. Cost was the major factor which prevented biologic use. Majority of the respondents used biologics in less than 2 cases per month. Secukinumab was the most common biologic used followed by etanercept. The factors which determined choice of biologics were convenience, cost, previous experience, co-morbid conditions and recommendations by an expert.
Limitations
A small sample size was the limitation of the study. Dermatologists who do not use biologics may be under-represented in the study.
Conclusions
Biologics are not used optimally by Indian dermatologists for management of psoriasis. The cost, fear of adverse effects, lack of awareness and inadequate felt need are major factors which prevent their regular use.
Keywords
Biologics
psoriasis
survey
Introduction
Psoriasis is a chronic T cell mediated disorder of unknown etiology, which affects skin, nail and joints. The evidence for systemic association of psoriasis with cardiac disease, metabolic syndrome, psychological morbidity and other co-morbidities is increasing.1 There has been a rapid increase in our understanding of the pathogenesis of psoriasis which has resulted in the development of targeted therapy.2
Biologics are protein molecules which target specific points in the immunopathogenesis of disease. Alefacept, in 2003, was the first biologic approved for the management of psoriasis. The earlier biologics like alefacept and efalizumab inhibited activation of T cells. Another approach targeted pro-inflammatory cytokines secreted by T cells. TNF alpha inhibitors like etanercept, infliximab, adalimumab and certolizumab pegol; IL-12/23 inhibitors like ustekinumab; IL-17 inhibitors like secukinumab, ixekizumab and broadalumab; IL-23 inhibitors like tildrakizumab, risankizumab and guselkumab are examples of this approach. There are biosimilars available for etanercept, infliximab and adalimumab.3 In our country, the biologics and biosimilars which are available for the management of psoriasis are etanercept, infliximab, adalimumab, itolizumab and secukinumab.
The use of biologics in psoriasis has advantages like rapid clearance and achieving 75%, 90% and 100% improvement in psoriasis area and severity index (PASI75/90/100) in a higher percentage of patients. They also cause less target organ damage as compared to conventional systemic therapy. Despite these advantages, it appears that biologics are not used optimally for the management of psoriasis in our country. The cost of biologics and non-availability of insurance cover are some of the factors for lesser usage of biologics. There are studies in western countries which report biologic usage based on disease registry or insurance claims database. Unfortunately in our country, we don’t have either and hence we thought of conducting a survey amongst prescribers to understand the pattern of biologic usage in their practice.
Methods
This was a cross-sectional questionnaire-based study. We designed a questionnaire with 30 questions after a focussed group discussion, involving three dermatologists and one epidemiologist. This questionnaire was sent to a group of 10 dermatologists and an epidemiologist to run a pilot test and to check for internal consistency. After taking inputs from experts, questions were re-designed and a final questionnaire with 24 questions was created. This questionnaire was again sent to the same set of experts for comments and after a final review, a survey was created on Survey MonkeyTM and sent via social media to approximately 1000 members of Indian Association of Dermatologists, Venereologists and Leprologists (IADVL). SPSS ver 25 was used for statistical analysis, Fisher’s exact test was used for categorical variables.
Results
The survey was sent via a link on email and social media groups. 310 members took the survey and 287 completed the survey. The average time to complete the survey was 3 minutes. Out of 287 completed responses, 115 (40%) dermatologists were practicing for <5 years, 75 (26%) for 5-10 years, 52 (18%) for 10-20 years and 45 (15%) for more than 20 years. The majority (193, 67%) of respondents were private practitioners. Majority (105, 36.6%) of them had an OPD of about 20-50 patients per day. Tablet methotrexate was the preferred systemic therapy by 263 (92%) respondents. Eighty-seven (30%) respondents did not use biologics in the management of psoriasis. The reasons for not using biologics are listed in Chart 1.
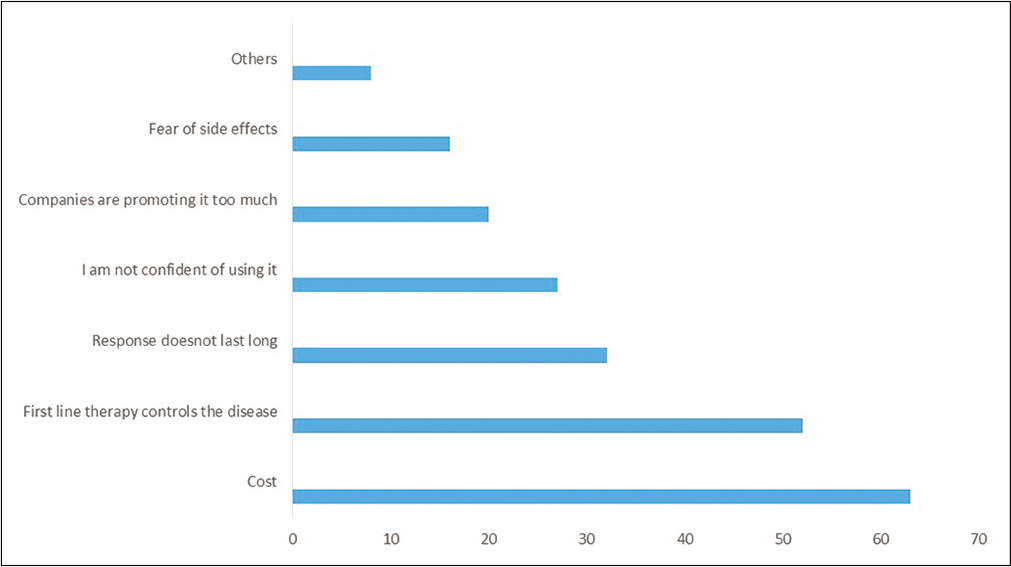
- Reasons for not using biologics
Two hundred respondents (70%) used biologics. Inadequate response to conventional systemic therapy was the most common reason for choosing biologics, followed by adverse effects of systemic therapy. Most respondents (140, 70%) used biologics in 2 or less cases per month, 52 (26%) used it in 2-5 cases and 8 (4%) respondents in more than 5 cases per month. The respondents who did not use biologics were more likely to be private practitioners (p = 0.029) and saw less severe cases as compared to those who use biologics (p < 0.001). The biologic use did not depend on years of practice (p = 0.793).
Secukinumab was the most common biologic used followed by etanercept. Most respondents felt secukinumab gave the fastest clearance followed by infliximab. The average duration of biologic use was for 3-6 months by 80 (40%) and 6-12 months by 54 (27%). The common reasons for stopping treatment with biologics were achieving remission in 100 (50%), cost in 48 (24%), inadequate response in 30 (15%) and adverse effects in 22 (11%).
Ninety-one (45%) respondents used biosimilars regularly because they were cheaper (81, 90%) and equally efficacious (22, 24%), while the rest were either unaware of biosimilars or were aware, but did not use them because of fear of inadequate response or higher adverse effects.
The factors which determined the choice of biologics in decreasing order were convenience of dosing (subcutaneous or intravenous), cost, previous experience, co-morbid conditions and recommendations by an expert. Most respondents screened for infections and metabolic syndrome, while 134 (67%) screened for psoriatic arthritis, 122 (61%) for congestive heart failure and 40 (20%) for inflammatory bowel disease (IBD) and neurological symptoms.
One hundred seventy six (88%) respondents felt that removing cost barrier would alter their pattern of biologic usage and they would use it more often. The source of information for biologics for most was from focused sessions on biologics in conferences followed by drug inserts. IADVL focused programs for biologics (IMPACT) was also a source of information for 30% of respondents.
Discussion
Biologics are a group of extremely effective drugs for the management of psoriasis and have been available for quite some time. They are very effective in clearing the disease; however, they are not used routinely because of various patient or prescriber related factors. In the survey conducted by Takeshita et al., in US Medicare population and in other studies on similar population, factors associated with higher likelihood of biologic use were comorbid ankylosing spondylitis, inflammatory bowel disease, psoriatic arthritis and renal disease. The unavailability of subsidy was associated with lower likelihood of biologic prescription. Most patients were prescribed subcutaneous self-administered biologics rather than intravenous biologic.4-7 Our study also shows the preference of prescribers for subcutaneous biologics by 192 (96%) respondents due to convenience.
Ara et al. conducted a survey of Spanish dermatologists and reported that 31% cases of moderate to severe psoriasis were treated with biologic therapy. Almost 28% of these patients required change in therapy due to inadequate response or adverse effects; in 60% cases, biologic was used as continuous treatment and the most important parameters were safety, long- term efficacy and tolerance.8 In this study, 30 (15%) respondents used biologics continuously while most stopped biologics after achieving clearance (3-6 months). The cost was the most important patient related factor for stopping biologics. The most important factors in choosing biologics were convenience, cost and previous experience of prescribers.
A systematic review concluded that psoriasis results in significant economic burden on patients and consequently, on the economy of the nation. Conventional systemic drugs like methotrexate are more cost effective and hence, are the first line agents. Biologics are expensive drugs, result in significant burden on healthcare cost and their usage is regulated in countries where state or insurance companies cover treatment costs. Since biologics for psoriasis are not covered under insurance in our country, it leads to out of pocket expenses and it is not surprising that cost remains a major hindrance in initiation and treatment continuation. A study conducted in Eastern European countries reported that higher per capita expenditure on health is associated with more biologic usage.9-11 In our study, cost was the foremost factor reported by 61 (70%) respondents for not using biologics and it is also one of the most important reasons to stop biologics prematurely. The majority felt that if this was not a consideration, they would use biologics in a greater number of patients.
The availability of biosimilars has the potential to increase usage of biologics by increasing access to them.12 In the present study, only 91 (44%) respondents used biosimilars regularly, while 22 (11%) were not aware of biosimilars. Lower cost of biosimilars was a major factor which prompted its usage by 82 (90%) respondents, while their poor efficacy was an important factor which prevented their use by 74 (37%) respondents. Secukinumab has become the most popular biologic in our country for psoriasis due to its convenience and faster disease clearance.13,14 In this study, 140 (70%) respondents had used more than 2 available biologics, but 134 (67%) respondents preferred secukinumab for the management of psoriasis followed by etanercept. The majority, 126 (63%) respondents felt that secukinumab resulted in the fastest clearance, while 46 (23%) felt the same about infliximab.
The guidelines suggest screening for active infection, congestive heart failure, neurological symptoms and IBD prior to starting biologic treatment.15,16 This study revealed that infections were a major concern for prescribers and screening was done by the majority whereas screening for other co-morbidities was suboptimal.
Limitations
Our study has several limitations. The information we gathered was based on a survey and there may be under representation of dermatologists who do not use biologics regularly or who do not use social media platforms. A small sample size is another limitation of our study.
Conclusion
Biologics are relatively safe and effective drugs for management of psoriasis. The cost is a major limitation. However, factors like fear of adverse effects and inadequate felt need also play an important role. In those who used biologics regularly, the usage was not optimal, they tended to stop the drug prematurely and screening protocols were inadequate. There is a need to increase awareness about biologics for better patient care. IADVL awareness sessions like IMPACT could help in increasing awareness about biologic usage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Psoriasis: Classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20:1475.
- [CrossRef] [PubMed] [Google Scholar]
- Joint AAD NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-72.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis in the US medicare population: Prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-63.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis treatment patterns with etanercept and adalimumab in a United States health plan population. J Dermatolog Treat. 2013;24:25-33.
- [CrossRef] [PubMed] [Google Scholar]
- Etanercept and adalimumab treatment patterns in psoriatic arthritis patients enrolled in a commercial health plan. Adv Ther. 2012;29:691-7.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate to severe psoriasis. J Manag Care Spec Pharm. 2015;21:201-9.
- [CrossRef] [PubMed] [Google Scholar]
- Opinion of Spanish dermatologists regarding the use of biologic therapy in patients with moderate to severe psoriasis. Actas Dermosifiliogr. 2011;102:706-16.
- [CrossRef] [Google Scholar]
- Comparison of the cost effectiveness of biologic drugs used for moderate to severe psoriasis treatment in the United States. J Dermatolog Treat. 2018;29:769-74.
- [CrossRef] [PubMed] [Google Scholar]
- Cost effectiveness of systemic treatments for moderate to severe psoriasis in the German health care setting. Arch Dermatol Res. 2016;308:249-61.
- [CrossRef] [PubMed] [Google Scholar]
- Economic burden of psoriasis in the United States: A systematic review. JAMA Dermatol. 2015;151:651-8.
- [CrossRef] [PubMed] [Google Scholar]
- Biosimilars for psoriasis: Worldwide overview of regulatory guidelines, uptake and implications for dermatology clinical practice. Br J Dermatol. 2017;177:1495-502.
- [CrossRef] [PubMed] [Google Scholar]
- Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate to severe psoriasis through 5 years of treatment (SCULPTURE Extension Study) J Eur Acad Dermatol Venereol. 2018;32:1507-14.
- [CrossRef] [PubMed] [Google Scholar]
- Real life efficacy and safety of secukinumab: A single center, retrospective observational study with 52 week follow up. Indian J Drugs Dermatol. 2019;5:14-8.
- [CrossRef] [Google Scholar]
- European S3 Guideline on the systemic treatment of psoriasis vulgaris Update Apremilast and Secukinumab EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2017;31:1951-63.
- [CrossRef] [PubMed] [Google Scholar]
- British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-36.
- [Google Scholar]






