Translate this page into:
Pemphigus occurring at tuberculin injection site: Role of cytokines in acantholysis
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Amrinder J Kanwar
Department of Dermatology, Venereology and Leprology Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh-160 012
India
| How to cite this article: Vinay K, Kanwar AJ, Saikia UN. Pemphigus occurring at tuberculin injection site: Role of cytokines in acantholysis. Indian J Dermatol Venereol Leprol 2013;79:539-541 |
Sir,
Pemphigus is a potentially fatal autoimmune blistering disorder. Until recently, the pathogenesis of pemphigus was described by the "desmoglein compensation theory" in which B-cells and anti-desmoglein (anti-Dsg) antibodies played a central role in inducing acantholysis. It is increasingly understood that desmoglein compensation theory is an over simplification of the pathogenic process and other autoantibodies, T-cells and cytokines play an important role in acantholysis. Lately Sardana et al., [1] have proposed an alternative hypothesis to desmoglein compensation theory to explain the disease process in pemphigus patients.
Recently we made an interesting clinical observation in three patients of pemphigus vulgaris (PV), diagnosed and confirmed by histological and immunopathological investigations. Patient 1 was a 40 year old female, who presented with 6 months history of oral erosions, and recent onset vesicles and erosions over trunk. She was diagnosed as PV and on clinical examination had an Ikeda severity score (ISS) of 9. Patient 2 was a 17 year old male patient of PV, who had failed to respond to 12 cycles of dexamethasone cyclophosphamide pulse therapy (DCP), and came with acute flare of disease activity and had an ISS of 11. Patient 3 was a 27 year old male and had relapse of the disease, after being successfully treated with DCP and had an ISS of 3. As a screening procedure for further therapy with rituximab, all patients were given 0.1 ml of standard dose of 5 tuberculin units, intradermally on the fore arm. Flaccid bulla was seen at the injection site of tuberculin test on day 3 in all cases [Figure - 1]. Tzanck smear from the lesion showed acantholytic cells, which raised the suspicion of koebnerization. A punch biopsy was obtained from the edge of these lesions and submitted for histopathological examination. All three biopsies showed suprabasal cleft containing acantholytic cells with tomb stone appearance of the basal cells [Figure - 2]. Tuberculin test was considered as inconclusive and patients were extensively evaluated (including contrast enhanced CT chest, bronchoalveolar lavage and early morning urine cultures) for any foci of active tuberculosis, with negative results. After a post treatment follow-up period of 16-24 months none of the patients have developed active tuberculosis.
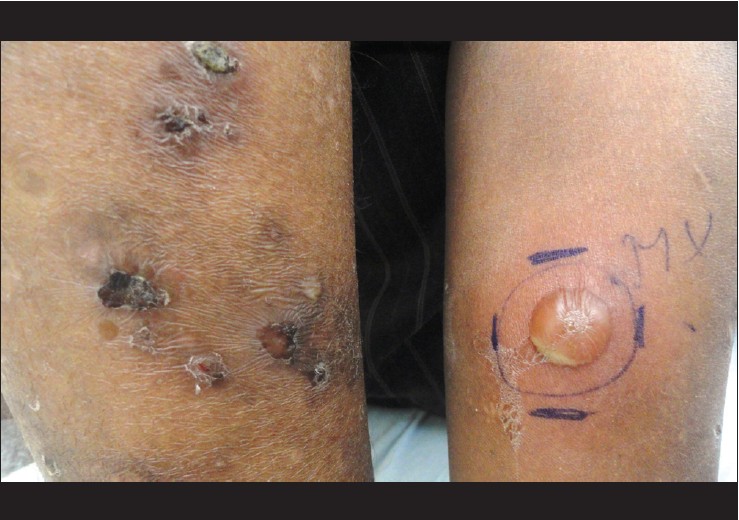 |
| Figure 1: Bulla at the injection site of tuberculin test three days after intradermal injection. Crusted erosions and a flaccid bulla can also be seen |
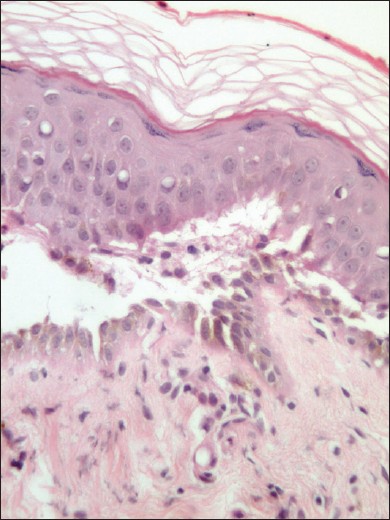 |
| Figure 2: Microphotography showing suprabasal cleft containing acantholytic cells and tombstone appearance of the basal cells (H and E × 200) |
It is essential to differentiate the phenomena noted in our patient from pathergy test described in neutrophilic dermatosis like Behçet′s disease. The skin lesions occurring after pathergy test is histologically distinct from the skin lesions occurring in the disease per se in contrast to Koebner phenomenon (KP). [2] Since all our patients showed distinctive histological features of PV a conclusion of KP secondary to tuberculin test was reached. Ueki described the pathogenesis of KP as a two step process. [3] A first non-specific inflammatory step and a second disease specific step. The first step includes production of various cytokines, stress proteins, and adhesion molecules. In the second step, there are disease specific reactions, including ones by T-cells, B-cells, autoantibodies, and immune deposits, under the restriction of genetic backgrounds. The exact pathogenesis of KP in pemphigus is largely unknown. Our cases are interesting in that koebnerization was seen after tuberculin test, which is a classical example of type IV hypersensitivity reaction. It is well known that Th1 cells and related cytokines are responsible for type IV hypersensitivity reaction, where as acantholysis in pemphigus is a Th2 mediated phenomena. Levels of Th2 cytokines IL-4, IL-6 and IL-10 are elevated in circulation in patients with active pemphigus. [4] These cytokines in turn prime B-cells to produce anti-Dsg antibodies. Various locally produced pro-inflammatory cytokines like IL-1α, IL-1β and TNF-α and activation of cell surface receptors like EGFR and mTOR also play a role in acantholysis in addition to steric hindrance caused by anti-Dsg antibodies. [5] These cytokines amplify the signals for acantholysis by modulating C3, plasminogen activator, and plasminogen activator inhibitor expression. [6] Tuberculin test is a memory T-cell mediated delayed hypersensitivity response. Intradermal injection of purified protein derivative is known to up regulate inflammatory cytokines IL-2, IFN-γ, IL-1α, IL-1β, IL-6 and TNF-α. [7] Study of kinetics of these cytokines showed that Th1 cytokines IL-2 and IFN-γ and T-cells secreting these cytokines reached a peak at 48 hours and declined by 72 hours. [7] Whereas macrophages and langerhans cells and their cytokines IL-1α, IL-1β, IL-6 and TNF-α reached a peak at 72 hours and was maintained till day 7. [7] It should be noted that these locally produced pro-inflammatory cytokines IL-1α, IL-1β, IL-6 and TNF-α play a major role in the sequence of acantholysis in pemphigus. [1] In our patients flaccid bulla was seen on day 3 of tuberculin injection when Th1 cytokines are declining and pro-inflammatory cytokines are at a peak. It is possible that the inflammatory milieu caused by tuberculin test was responsible for the KP seen in our patients. However, lack of use of negative control for tuberculin test limits a firm conclusion on this hypothesis. These patients gave us a clinical opportunity to study the facilitative role played by pro-inflammatory cytokines (analogues to non-specific inflammatory step in KP pathogenesis) in inducing skin lesion of pemphigus in patients with pathogenic anti-Dsg antibodies (analogues to the specific step of KP pathogenesis). It further supports the pathogenic role played by T-cells and cytokines in pemphigus as proposed by Sardana et al.,[1] TNF-α inhibitors like infliximab, etanercept and adalimumab has been used in the treatment of pemphigus. In future, therapies directed against other cytokines like IL-1 and IL-6 may help in more effective management of patients with immunobullous disorders.
| 1. |
Sardana K, Garg VK, Agarwal P. Is there an emergent need to modify the desmoglein compensation theory in pemphigus on the basis of ELISA data and alternative pathogenic mechanisms? Br J Dermatol. 2013;168:669-74.
[Google Scholar]
|
| 2. |
Varol A, Seifert O, Anderson CD. The skin pathergy test: Innately useful? Arch Dermatol Res 2010;302:155-68.
[Google Scholar]
|
| 3. |
Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev 2005;4:219-23.
[Google Scholar]
|
| 4. |
Giordano CN, Sinha AA. Cytokine networks in Pemphigus vulgaris: An integrated viewpoint. Autoimmunity 2012;45:427-39.
[Google Scholar]
|
| 5. |
Grando SA. Pemphigus autoimmunity: Hypotheses and realities. Autoimmunity 2012;45:7-35.
[Google Scholar]
|
| 6. |
Seko Y, Cole S, Kasprzak W, Shapiro BA, Ragheb JA. The role of cytokine mRNA stability in the pathogenesis of autoimmune disease. Autoimmun Rev 2006;5:299-305.
[Google Scholar]
|
| 7. |
Chu CQ, Field M, Andrew E, Haskard D, Feldmann M, Maini RN. Detection of cytokines at the site of tuberculin induced delayed type hypersensitivity in man. Clin Exp Immunol 1992;90:522-9.
[Google Scholar]
|
Fulltext Views
2,421
PDF downloads
1,817





