Translate this page into:
Percutaneous ethanol injection as a promising and minimally invasive treatment for axillary osmidrosis: Double-blinded randomized controlled trial
2 Skin and Stem Cell Research Center, Tehran University of Medical Sciences, Tehran, Iran
Correspondence Address:
Masoom Shahbazi
Department of Dermatology, Al Zahra Hospital, Soffeh Blvd, Isfahan University of Medical Sciences, Isfahan
Iran
| How to cite this article: Asilian A, Shahbazi M, Abtahi-Naeini B, Poostiyan N, Nilforoushzadeh MA. Percutaneous ethanol injection as a promising and minimally invasive treatment for axillary osmidrosis: Double-blinded randomized controlled trial. Indian J Dermatol Venereol Leprol 2018;84:157-162 |
Abstract
Background: Axillary osmidrosis is a common problem with a strong negative impact on the professional and social quality of life. Several options are available for its treatment. But there are no treatment guidelines. The objective of this study was to evaluate efficacy and safety of percutaneous ethanol injection for treatment of axillary osmidrosis.Methods: A randomized, double-blind, placebo-controlled clinical trial to assess clinical efficacy and postoperative complications of percutaneous ethanol injection was performed among 60 patients (12–35 years of age) with axillary osmidrosis. The active agent used in the experimental group (n = 30) was sterile 90% ethanol and the placebo used in the control group (n = 30) was sterile normal saline administered in an identical syringe. The results of malodor elimination were graded by the patients as excellent, good, fair, and poor. All patients were followed-up for 10 months.
Results: Malodor elimination was graded as good by 15 (50%) patients treated with percutaneous ethanol injection. A significant difference in the improvement of axillary osmidrosis was found between the experimental and control groups (P < 0.001). The most common post-procedure complication was transient subjective skin stiffness in the experimental group, which regressed spontaneously. There were no serious permanent side effects.
Limitations: Relatively short duration of follow-up; and lack of histopathological evidence of destruction of the apocrine glands after treatment in most patients.
Conclusions: Percutaneous ethanol injection is an effective and safe treatment method for axillary osmidrosis and does not have permanent side effects.
Introduction
Axillary osmidrosis is characterized by an excessive, unpleasant odor that originates from the apocrine glands in the axillary area. Axillary osmidrosis with and without hyperhidrosis is a common condition with significant impact on the quality of life of affected persons.[1],[2] The purpose of treatment is to destroy sweat glands with minimal effect on the appearance of axillary area.[3],[4] Many treatment modalities have been developed for axillary osmidrosis.[5] These are classified as surgical and nonsurgical. Surgical interventions include removal of axillary tissue using lasers; use of ultrasonic devices or endoscope; liposuction; and manual subcision.[3],[6],[7],[8],[9]
Surgical treatment in which the subcutaneous tissue including the apocrine glands is removed provides the best results. However, it carries a high risk of adverse effects, such as hemorrhage, hematoma, infection, pain, scarring and limitation of movement.[6],[8] For cosmetic reasons, minimally invasive procedures are preferred nowadays. Ethanol is a primary sclerosing agent considered as a therapeutic option in axillary osmidrosis.[10] Percutaneous ethanol injection is currently the first-choice treatment of cystic nodules relapsed after diagnostic evacuation. Moreover, it is an alternative treatment for patients with nodular lesions who have contraindications for surgery and for those who carry a high risk from surgery.[11]
A recent study suggested that percutaneous ethanol injection is effective in elimination of malodor in axillary osmidrosis.[10] But this study was not blinded and did not have a control group. Here we report the results of a randomized, double-blind, placebo-controlled trial for evaluating the efficacy and safety of percutaneous ethanol injection in the treatment of axillary osmidrosis.
Method
Participants
We performed a prospective, parallel group, randomized, clinical trial to assess clinical efficacy and postoperative complications of percutaneous ethanol injection in the treatment of axillary osmidrosis at Al-Zahra university hospital, Isfahan, Iran.
Considering type I error (alpha) as 0.05, power as 80% and an expected difference of 30% in response rate between the groups, sample size was calculated as 30 in each group.
From April 2014 to April 2015, 60 patients (age range 12 to 35 years) with moderate to severe axillary osmidrosis (grade 3 or 4), according to the osmidrosis grading system,[13] were enrolled in the study. Exclusion criteria included pregnancy, breast feeding, previous axillary operation, major co-morbidities, treatment with botulinum toxin or radiosurgery during the previous four months and active infection of the axillae. The study protocol was approved by the institutional review board of Isfahan University of Medical Sciences. Written informed consent was obtained from all the participants. We recorded age and sex, age of onset of disease, and family history of the participants.
Participants were divided into two groups in a 1:1 ratio using random allocation software for parallel group randomized trials. The members of the study arm were injected percutaneously in the axillae with sterile 90% ethanol (Sina FarimanCo., Mashhad, Iran) and those in the placebo group with sterile normal saline (0.9% sodium chloride, PLC, Tehran, Iran). Both preparations were administered using identical 2.5 mL syringes.
Participants and the members of the dermatologic surgery team who injected the medication were unaware of the contents of the injection. Only one of the co-investigators, who generated the random allocation sequence and enrolled participants, was not blinded in the study.
Surgical procedures
All procedures were performed by the same surgical team. Patients were placed in supine position in the outpatient operating room, with their arm abducted to approximately 100°. Excessive abduction was avoided to prevent brachial plexus injury.[14],[15] To reduce uneven distribution of medication, a grid pattern consisting of 1 × 1 cm squares was drawn on both axillae preoperatively [Figure - 1]. This was followed by iodine starch test (Minor's test) to identify the preoperative sweating pattern and thereby to determine the surface area required for the operation.[16]
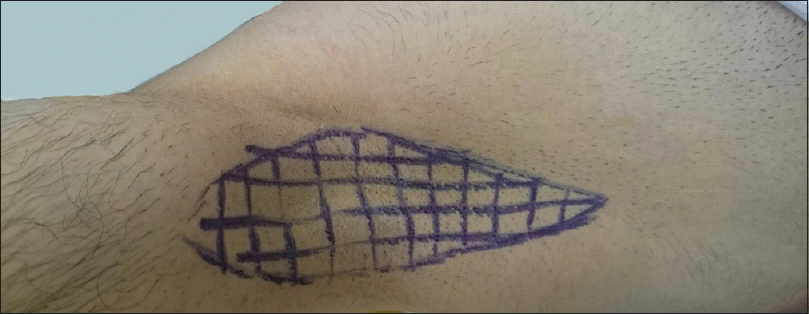 |
| Figure 1: Grid pattern of 1 × 1 cm squares drawn on the axillae preoperatively |
Lidocaine 2% (Pasteur Institute, Iran) diluted to 0.25% by mixing with normal saline was used for local anaesthesia. Thirty mL of this preparation was infiltrated subcutaneously in each axilla. The volume of injection was calculated based on the volume of the target tissue.[10] One milliliter of ethanol or placebo per 1 cm [3] of tissue was injected into the dermo-subdermal junction (superficial subcutaneous layer) using a 2.5 mL syringe and 30-G needle.
In the experimental group, sterile 90% ethanol was injected on the marked area. The injection was stopped when the content of the syringe was completely used up or when the patient complained of severe pain. In the control group, sterile normal saline (0.9% sodium chloride, PLC, Tehran, Iran) was injected on the marked area. The site was dressed with a thin gauze after injection and no bandage was used. Entire procedure, barring the contents of syringes was same in both groups.
Outcome assessment
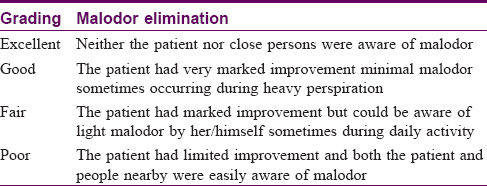
Primary overall efficacy determined was a change in axillary malodor. The results of malodor elimination were graded by the patients as excellent, good, fair, and poor [17] [Table - 1]. Moderate osmidrosis was defined as malodor that could be identified by the patient but could not be detected by others at a distance of 1.5 m from the patient and accompanied by a little perspiration, and severe malodor and sweating could be detected easily from the body by others at a distance of 1.5 m during normal daily activity.[9]
The final results of clinical efficacy were assessed by the patients themselves, their family members and/or close friends and blinded investigators. Also we reviewed the clinical records and interviewed the patients individually during the follow-up visit or via telephone. All patients were followed up for ten months. The secondary outcome determined was satisfaction of patients after 10 months of treatment. Satisfaction of patients was assessed by a visual analogue scale (VAS) from 0 to 10 (0, no satisfaction; 10, best possible satisfaction).[18]
Histologic examination
Preoperative and postoperative histopathological findings of axillary tissue were reviewed in two patients who gave consent to a histologic examination. The axillary specimen was obtained by 4-mm punch biopsy at the center of axilla.
Postoperative complication evaluation
Symptoms of all important side-effects and postoperative complications including ecchymosis, induration, haematoma or seroma, infection, skin necrosis, contracture, and nerve damage were explained to the patients before and during assessment and were evaluated at day one, seven, 30 and then monthly up to 6 months by one of blinded co-investigator. Information regarding recurrence of axillary osmidrosis was gathered.
Statistical analysis
Data analyses were performed using the Statistical Package for Social Sciences (SPSS©, Chicago, IL, USA) for Windows®, Version 16.0 for 30 participants in each group. A P value <0.05 was considered to be statistically significant.
Disease characteristics were compared between the two groups using Chi-square for categorical variables, Mann–Whitney U test for nonparametric ordinal variables, and independent-samples t-tests for parametric variables.
The changes from the baseline to the end of study period within each group were tested using Wilcoxon Signed Rank test for nonparametric variables. Analysis in subgroups (responder and nonresponder participants) was done using Chi-square test and independent-samples t-tests.
Results
Demographic data
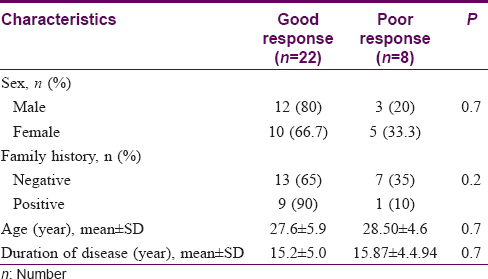
All 30 patients in each group completed the study and were considered for final analysis. All patients were followed up for 10 months. Mean age of patients was 27.9 ± 5.5 years in the experimental group and 27.0 ± 4.9 years in the control group. (P = 0.50). Other patient characteristics are summarized in [Table - 2].

Malodor elimination grading
Using Mann–Whitney U-test, a significant difference in the improvement of osmidrosis [Figure - 2] was found between the experimental and control groups (P < 0.001).
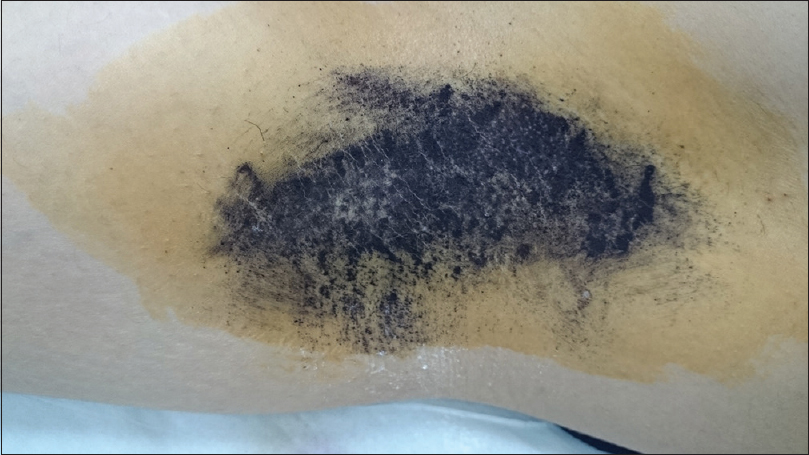 |
| Figure 2a: Iodine starch test. Minor's solution and starch applied on the axilla of a 33-year-old female with Grade 3 osmidrosis showing preoperative sweating pattern |
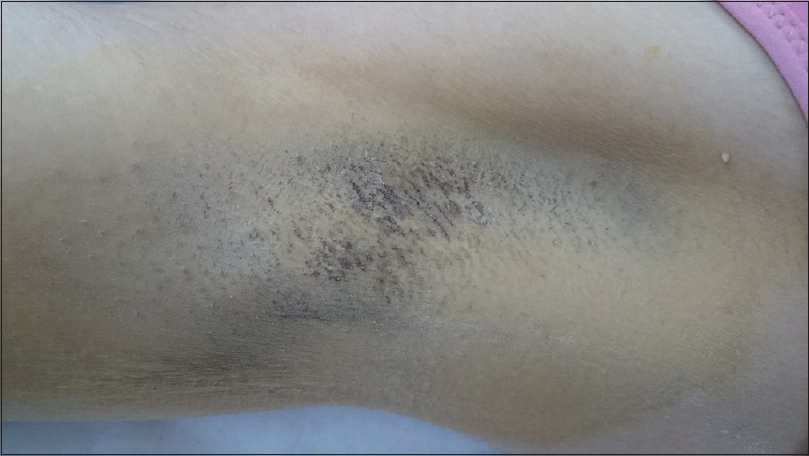 |
| Figure 2b: Ten months after percutaneous ethanol injection showing marked clinical improvement |
[Table - 3] provides a comparison of demographic characteristics of patients who responded well and those who did not in the experimental group.
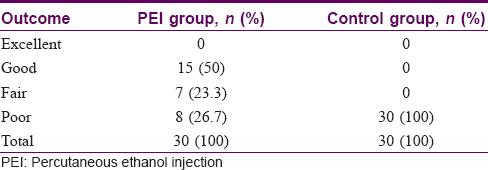
Approximately 75% of patients reported fair to good improvement in the experimental group [Figure - 3] and [Table - 4].
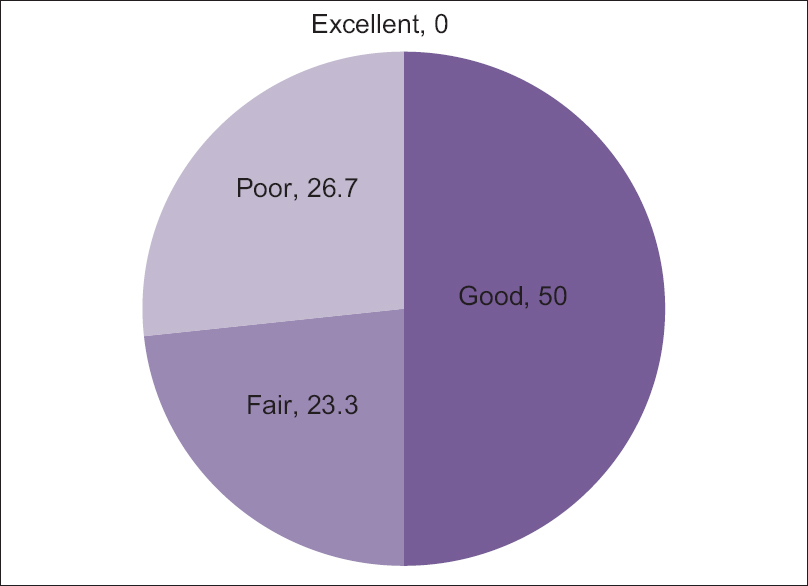 |
| Figure 3: Results of clinical efficacy after percutaneous ethanol injection |
Histopathological findings
Biopsy of the axillary skin and subcutaneous tissue was done in two patients before and after treatment. Pretreatment histopathology showed an increased number of apocrine glands. Post-treatment histopathology showed that most of the subcutaneous layer and deep dermis were destroyed. Remnant sweat glands were markedly destroyed.
Patient satisfaction
Mean VAS score of patient's satisfaction was 4.8 ± 3.4 in the experimental group. Patients who who reported fair to good response were more satisfied (VAS = 6.4 ± 2.5) with the outcome of the procedure compared to those who reported poor response (VAS = 0.5 ± 0.92 P< 0.001).
Side-effects
There were no serious permanent complications such as skin necrosis, infection, massive hematoma, or seroma. There were no side effects attributed to ethanol toxicity. The most common adverse effect was transient subjective skin stiffness in the experimental group. These side effects were mild, tolerable, and disappeared completely within 3–4 weeks after treatment. Details of complications in the treatment group are listed in [Table - 5].
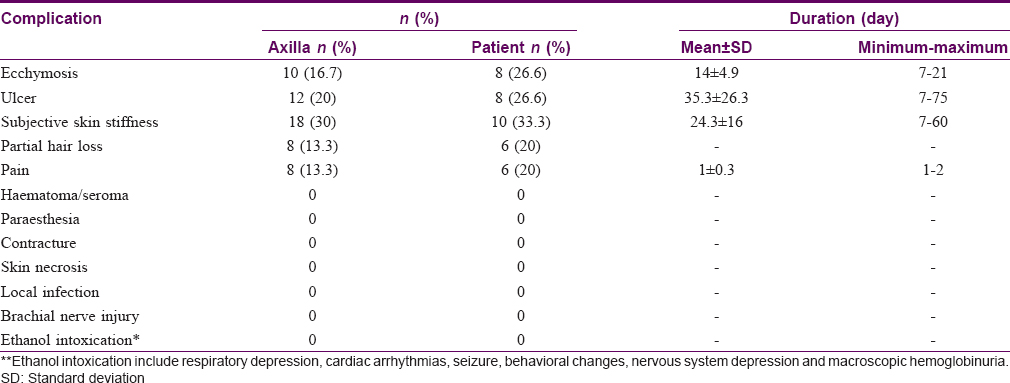
Ten (16.7%) and 12 (20%) of 60 axillae in the experimental group experienced ecchymosis [Figure - 4] and mild to moderate ulceration [Figure - 5] and received conservative management as treatment. Full recovery took approximately 35 days. Although the frequency of complications in the experimental group was significantly higher than that in the control group (P < 0.01), all complications were transient, could be controlled well and left no permanent sequelae.
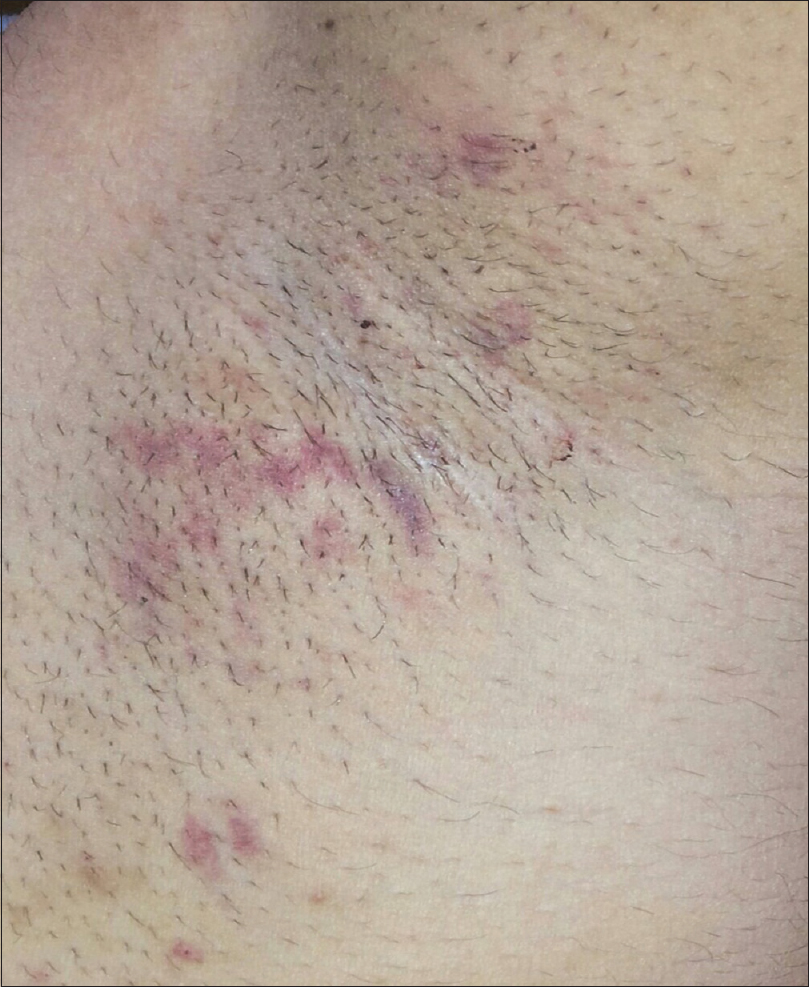 |
| Figure 4: Ecchymosis following percutaneous ethanol injection in a 24-year-old male |
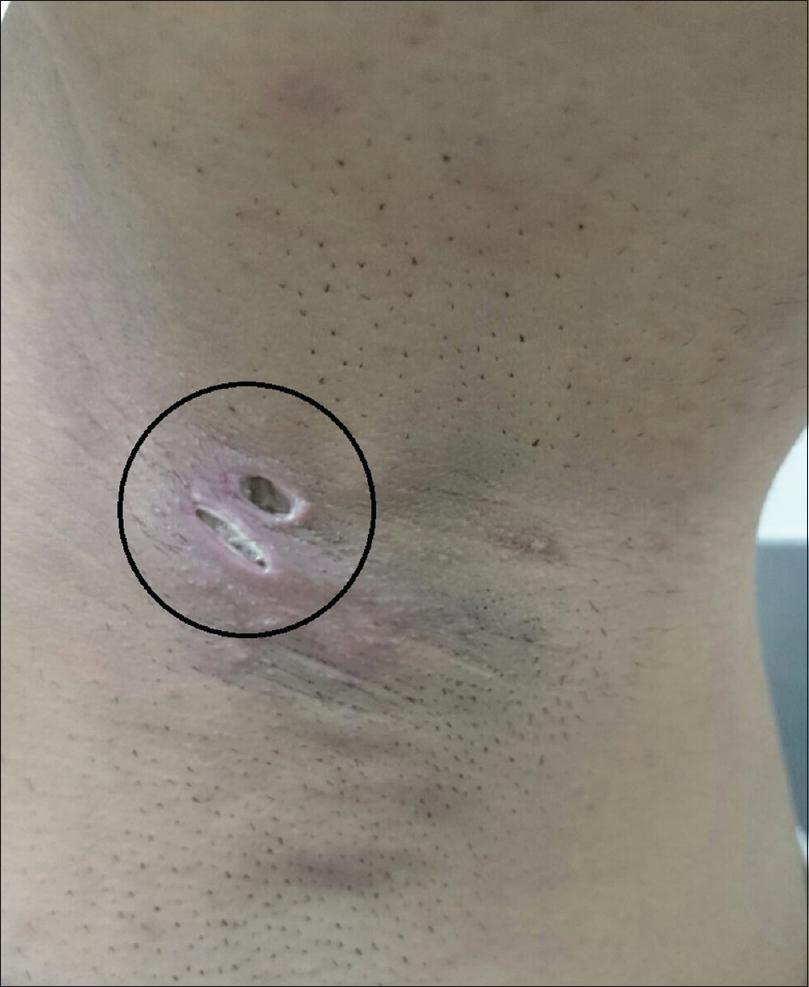 |
| Figure 5: Ulceration five days after percutaneous ethanol injection in a 30-year-old male |
Discussion
Percutaneous ethanol injection is an effective and safe treatment for axillary osmidrosis without permanent complications. Sclerosing properties of ethanol have been known for many years. Percutaneous ethanol injection is an accepted method of treatment of hepatocellular carcinoma.[19] It is also used in the treatment of thyroid and parathyroid disease.[20],[21] The mechanism of action of ethanol is related to cellular dehydration, coagulation necrosis, thrombosis, and vascular occlusion.[22]
Instead of removing unwanted tissue surgically, ethanol causes destruction of sweat glands. Histopathological examination of thyroid nodules after percutaneous ethanol injection confirmed that ethanol injection was successful in destroying the tissue, resulted in atrophy or decrease in tissue volume, and thus in the elimination of hyperactivity.[24] Ethanol is injected into the subcutaneous layer near the interface of the dermis, where the apocrine and eccrine glands are located.[25] Apocrine glands are more widely distributed in the subcutaneous tissue than in dermis. Patients with axillary osmidrosis typically have many glandular tissues with thick layers.[9]
They have more numerous and larger apocrine glands than normal persons. The ratio of apocrine to eccrine glands in the axillary skin is approximately 1:8 in normal persons, but is approximately 1:0.8 in patients with axillary osmidrosis.[24] Percutaneous ethanol injection causes necrosis of the apocrine glands, while the superficial skin and underlying vital structures are left intact.[11],[24]
There are only a few publications about treatment of axillary osmidrosis with percutaneous ethanol injection. Han and Li treated 165 patients with bilateral axillary osmidrosis with percutaneous ethanol injection and followed them up for a period of 14.5 months. Majority of these patients showed excellent to good results.[10] In our study, 50% of the patients treated with percutaneous ethanol injection reported good to excellent improvement with no severe complications. Difference in the findings of our study compared to the study by Han and Li may be attributed to differences in personal, racial and ethnic characteristics of the patients.
Compared with the study of Han and Li, none of our participants developed axillary skin necrosis due to refuse of epinephrine in solutions of local anesthesia. Han and Li used a solution of 1 in 200,000 epinephrine in the early stages of their study and two patients developed necrosis of the entire axillary skin on both sides.[10]
The histopathologic findings of our study indicate that percutaneous ethanol injection.reduces sweating by causing direct damage of apocrine glands. However, the existence of apocrine glands even after treatment indicates that recurrence is possible on long-term follow-up. This may necessitate additional treatment sessions.[26],[27]
Our study underlines the efficacy of percutaneous ethanol injection.in the treatment of axillary osmidrosis clinically and histopathologically. Based on these results, percutaneous ethanol injection can be considered as a promising, minimally invasive and effective treatment for axillary osmidrosis.
Conclusion
Percutaneous ethanol injection is an effective alternative method for treatment of axillary osmidrosis. Complications are mild to moderate but transitory and self-limited. Our study is a primary study for evaluation of clinical efficacy and safety of percutaneous ethanol injection. Comparative studies are needed to confirm if the response is sustained for a long time.
Limitations
There are several limitations to our study, including the relatively short duration of follow-up and the lack of histopathological evidence in all subjects confirming destruction of the apocrine glands after percutaneous ethanol injection. Another limitation of our study was lack of more objective methods for assessment of clinical efficacy.
Acknowledgment
We would like to acknowledge the patients for their participation in the study, and Mrs. Nekoei for her technical help during the study.
Financial support and sponsorship
This study was supported financially by Skin Disease and Leishmaniasis Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Huang YH, Yang CH, Chen YH, Chen CH, Lee SH. Reduction in osmidrosis using a suction-assisted cartilage shaver improves the quality of life. Dermatol Surg 2010;36:1573-7.
[Google Scholar]
|
| 2. |
Abtahi-Naeini B, Naeini FF, Adibi N, Pourazizi M. Quality of life in patients with primary axillary hyperhidrosis before and after treatment with fractionated microneedle radiofrequency. J Res Med Sci 2015;20:631-5.
[Google Scholar]
|
| 3. |
Kao TH, Pan HC, Sun MH, Chang CS, Yang DY, Wang YC. Upper thoracic sympathectomy for axillary osmidrosis or bromidrosis. J Clin Neurosci 2004;11:719-22.
[Google Scholar]
|
| 4. |
Qian JG, Wang XJ. Radical treatment of axillary osmidrosis by subdermal excision of apocrine glands: A prospective study in 31 cases. J Plast Reconstr Aesthet Surg 2006;59:860-4.
[Google Scholar]
|
| 5. |
Perng CK, Yeh FL, Ma H, Lin JT, Hwang CH, Shen BH, et al. Is the treatment of axillary osmidrosis with liposuction better than open surgery? Plast Reconstr Surg 2004;114:93-7.
[Google Scholar]
|
| 6. |
Chen YT, Shih PY, Chen HJ, Chen TJ. Treatment of axillary osmidrosis: A comparison between subcutaneous laser and superficial liposuction curettage. J Eur Acad Dermatol Venereol 2015;29:2019-23.
[Google Scholar]
|
| 7. |
Wang C, Wu H, Du F, Le S, Zheng S. Axillary osmidrosis treatment using an aggressive suction-curettage technique: A clinical study on paired control. Aesthetic Plast Surg 2015;39:608-15.
[Google Scholar]
|
| 8. |
Liu Q, Zhou Q, Song Y, Yang S, Zheng J, Ding Z. Surgical subcision as a cost-effective and minimally invasive treatment for axillary osmidrosis. J Cosmet Dermatol 2010;9:44-9.
[Google Scholar]
|
| 9. |
Kim D, Kim J, Yeo H, Kwon H, Son D, Han K. Treatment of axillary osmidrosis using a subcutaneous pulsed Nd-YAG laser. Arch Plast Surg 2012;39:143-9.
[Google Scholar]
|
| 10. |
Han X, Li F. Percutaneous ethanol injection for the treatment of axillary osmidrosis. Clin Exp Dermatol 2013;38:484-8.
[Google Scholar]
|
| 11. |
Perez CL, Fighera TM, Miasaki F, Mesa Junior CO, Paz Filho GJ, Graf H, et al. Evaluation of percutaneous ethanol injections in benign thyroid nodules. Arq Bras Endocrinol Metabol 2014;58:912-7.
[Google Scholar]
|
| 12. |
Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med 2004;351:1764-71.
[Google Scholar]
|
| 13. |
Park YJ, Shin MS. What is the best method for treating osmidrosis? Ann Plast Surg 2001;47:303-9.
[Google Scholar]
|
| 14. |
Arneja JS, Hayakawa TE, Singh GB, Murray KA, Turner RB, Ross LL, et al. Axillary hyperhidrosis: A 5-year review of treatment efficacy and recurrence rates using a new arthroscopic shaver technique. Plast Reconstr Surg 2007;119:562-7.
[Google Scholar]
|
| 15. |
Rho NK, Shin JH, Jung CW, Park BS, Lee YT, Nam JH, et al. Effect of quilting sutures on hematoma formation after liposuction with dermal curettage for treatment of axillary hyperhidrosis: A randomized clinical trial. Dermatol Surg 2008;34:1010-5.
[Google Scholar]
|
| 16. |
Fatemi Naeini F, Pourazizi M, Abtahi-Naeini B, Nilforoushzadeh MA, Najafian J. A novel option for treatment of primary axillary hyperhidrosis: Fractionated microneedle radiofrequency. J Postgrad Med 2015;61:141-3.
[Google Scholar]
|
| 17. |
Lee D, Cho SH, Kim YC, Park JH, Lee SS, Park SW. Tumescent liposuction with dermal curettage for treatment of axillary osmidrosis and hyperhidrosis. Dermatol Surg 2006;32:505-11.
[Google Scholar]
|
| 18. |
Fatemi Naeini F, Abtahi-Naeini B, Pourazizi M, Nilforoushzadeh MA, Mirmohammadkhani M. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: A sham control study. Australas J Dermatol 2015;56:279-84.
[Google Scholar]
|
| 19. |
Zhang Y, Zhang M, Fan X. Serum concentrations of matrix metalloproteinase-9 and vascular endothelial growth factor affect the prognosis of primary hepatic carcinoma patients treated with percutaneous ethanol injection. Int J Clin Exp Med 2015;8:16036-42.
[Google Scholar]
|
| 20. |
Bennedbaek FN, Karstrup S, Hegedüs L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. Eur J Endocrinol 1997;136:240-50.
[Google Scholar]
|
| 21. |
American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214.
[Google Scholar]
|
| 22. |
Crescenzi A, Papini E, Pacella CM, Rinaldi R, Panunzi C, Petrucci L, et al. Morphological changes in a hyperfunctioning thyroid adenoma after percutaneous ethanol injection: Histological, enzymatic and sub-microscopical alterations. J Endocrinol Invest 1996;19:371-6.
[Google Scholar]
|
| 23. |
Pomorski L, Bartos M. Histologic changes in thyroid nodules after percutaneous ethanol injection in patients subsequently operated on due to new focal thyroid lesions. APMIS 2002;110:172-6.
[Google Scholar]
|
| 24. |
Bang YH, Kim JH, Paik SW, Park SH, Jackson IT, Lebeda R. Histopathology of apocrine bromhidrosis. Plast Reconstr Surg 1996;98:288-92.
[Google Scholar]
|
| 25. |
Naeini FF, Saffaei A, Pourazizi M, Abtahi-Naeini B. Histopathological evidence of efficacy of microneedle radiofrequency for treatment of axillary hyperhidrosis. Indian J Dermatol Venereol Leprol 2015;81:288-90.
[Google Scholar]
|
| 26. |
Grice K, Sattar H, Baker H. The effect of ambient humidity on transepidermal water loss. J Invest Dermatol 1972;58:343-6.
[Google Scholar]
|
| 27. |
Abtahi-Naeini B, Naeini FF, Saffaei A, Behfar S, Pourazizi M, Mirmohammadkhani M, et al. Treatment of primary axillary hyperhidrosis by fractional microneedle radiofrequency: Is it still effective after long-term follow-up? Indian J Dermatol 2016;61:234.
[Google Scholar]
|
Fulltext Views
4,108
PDF downloads
1,849





