Translate this page into:
Peripheral T-cell lymphoma at the injection site of influenza vaccination
2 Department of Dermatology, Outpatient Clinic, Changhai Hospital, Second Military Medical University, Shanghai, China
3 Department of Pathology, Changhai Hospital, Second Military Medical University, Shanghai, China
Correspondence Address:
Jun Gu
Department of Dermatology, Changhai Hospital, Second Military Medical University, Shanghai
China
Miao-xia He
Department of Pathology, Changhai Hospital, Second Military Medical University, Shanghai
China
| How to cite this article: Bi Xl, Liu Yf, He Mx, Gu J. Peripheral T-cell lymphoma at the injection site of influenza vaccination . Indian J Dermatol Venereol Leprol 2014;80:526-529 |
Abstract
Pseudolymphomas or B-cell lymphoma at the vaccination site have been reported by several authors. However, onset of cutaneous T-cell lymphoma with cytotoxic features is a rare complication of vaccination. We report a 27-year-old man who developed a nodule and ulcer that arose at the site of injection of influenza vaccine. The neoplastic cells reacted positively for CD56, CD3, CD2, perforin, and granzyme B, but negatively for CD4, CD8, CD10, CD19, CD30, CD34, CD79, and betaF1. Molecular studies showed T-cell receptor γ (TCR-γ) chain monoclonal rearrangement. A diagnosis of peripheral T-cell lymphoma, not otherwise specified (NOS) was established. The patient had high fever, progressive liver dysfunction and a rapid fatal evolution.INTRODUCTION
Vaccination is the most effective means to prevent or to reduce illness and death from pandemic influenza infection. However, vaccine reactions and immunogenicity should also be considered in anyone who receives the pandemic or seasonal influenza vaccine. [1] Local vaccination reactions include mild erythema, edema, pain, induration, papules, or subcutaneous nodules. These side effects usually do not persist for a long time. Pseudolymphomas and B-cell lymphoma at the vaccination site have been reported. [2],[3] We report a young man who developed peripheral T-cell lymphoma at the site of influenza vaccination.
CASE REPORT
A 27-year-old man developed a nodule and ulcer at the inoculation site of influenza vaccine on the left upper arm. Within a few days after the injection, the nodule grew to 2 cm in diameter. The patient was seen at a peripheral hospital and treated with topical antibiotics, but the lesion continued to increase in size and gradually became more indurated and ulcerated [Figure - 1]a. There was no regional lymphadenopathy. Five months after the initial presentation, debridement of the lesion was done. Histopathologic examination of the specimen showed a granulomatous and mixed cellular infiltration. Hence, he was diagnosed with granulomatous reaction following vaccination. However, in the following 2 months, the lesion continued to enlarge while the patient became febrile along with the appearance of numerous red plaques on his trunk and limbs [Figure - 1]b. He was then referred to our hospital for evaluation of the enlarging ulcer, plaques, and fever.
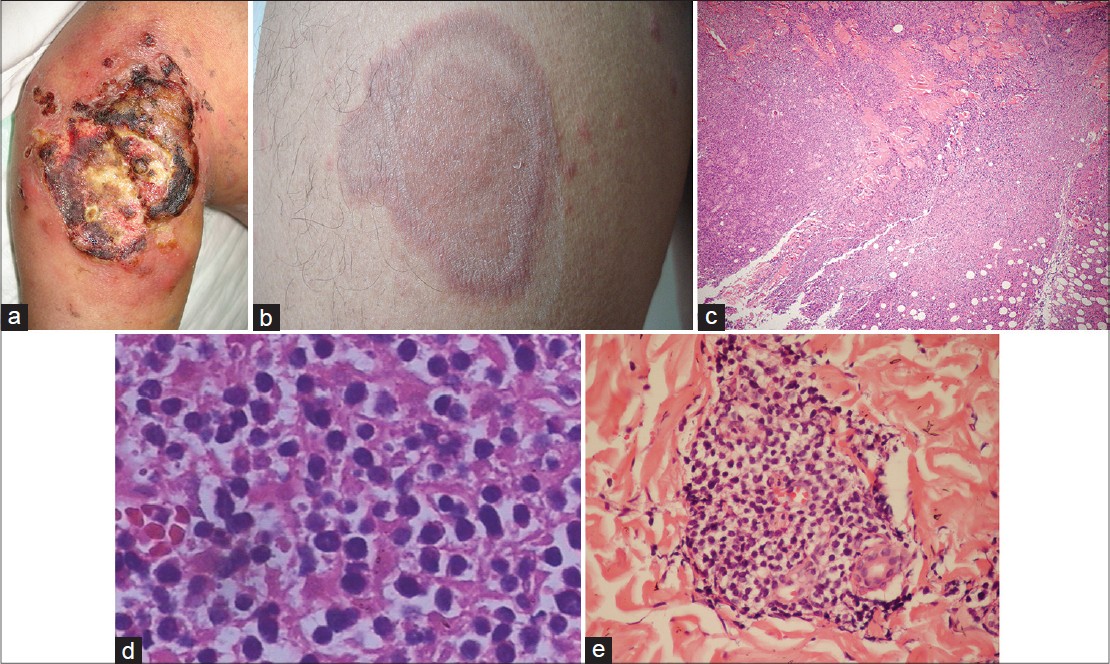 |
| Figure 1: Lymphoma manifesting as ulcer on the left arm and multiple plaques on the trunk and limbs. (a) 8 × 10 cm ulcerated tumor present at the inoculation site of vaccination. (b) Multiple red infiltrating plaques on the trunk and limb. (c) Biopsy of the necrotic tumor on the left arm reveals atypical lymphoid cells throughout the dermis extending into subcutaneous fat with an angiocentric pattern and extensive necrosis (hematoxylin and eosin (H and E), ×40). (d) Polymorphous lymphoid infiltrate (H and E, ×400). (e) Mixed infiltrates of large and small lymphocytes with irregular nuclei around dermal appendages and blood vessels in a biopsy of the plaque on the trunk (H and E, ×200) |
Skin biopsies for histopathology, immunohistochemistry, and molecular analyses were obtained from the ulcerated tumor on the left arm and a plaque on the trunk. The biopsy from the left arm showed an ulcerated epidermis and infiltration of atypical lymphoid cells along the full thickness of the dermis which extended into the subcutaneous fat with an angiocentric pattern [Figure - 1]c and d. Re-evaluation of the initial biopsy also revealed infiltration of atypical lymphocytes. The biopsy from the red plaque on the trunk showed infiltration of small to large lymphoid cells with irregular nuclei around dermal appendages and blood vessels [Figure - 1]e. Immunohistochemistry on the specimen from the left arm lesion showed the majority of the lymphoid cells to be positive for CD3, CD7, and CD2; but negative for CD4, CD8, CD10, CD19, CD30, CD34, CD79, Bcl-2, and Bcl-6. The pan B-cell marker CD20 was negative and betaF1 staining was negative on paraffin sections. Natural killer (NK) lineage marker CD56 and the cytotoxic effectors granzyme B and perforin were strongly expressed. The Ki-67 proliferation index was relatively high [Figure - 2]. Infiltrating cells of the trunk biopsy were positive for CD3, CD2, and CD56, and negative for CD4, CD8, CD10, CD19, CD30, and CD34, and expressed granzyme B and perforin weakly. In situ hybridization with fluorescein conjugated ribonucleic acid (RNA) probe for the small Epstein- Barr virus (EBV)-encoded RNA 1 and 2 (EBER1/2) showed no evidence of EBV infection. Using a primer for the V and J regions of T-cell receptor (TCR) γ chain, polymerase chain reaction (PCR) showed monoclonal rearrangement, indicating the presence of a monoclonal T-cell population. T-cell lymphoma cell line Jurkat and B-cell lymphoma cell line Raji were used as positive controls [Figure - 2]e.
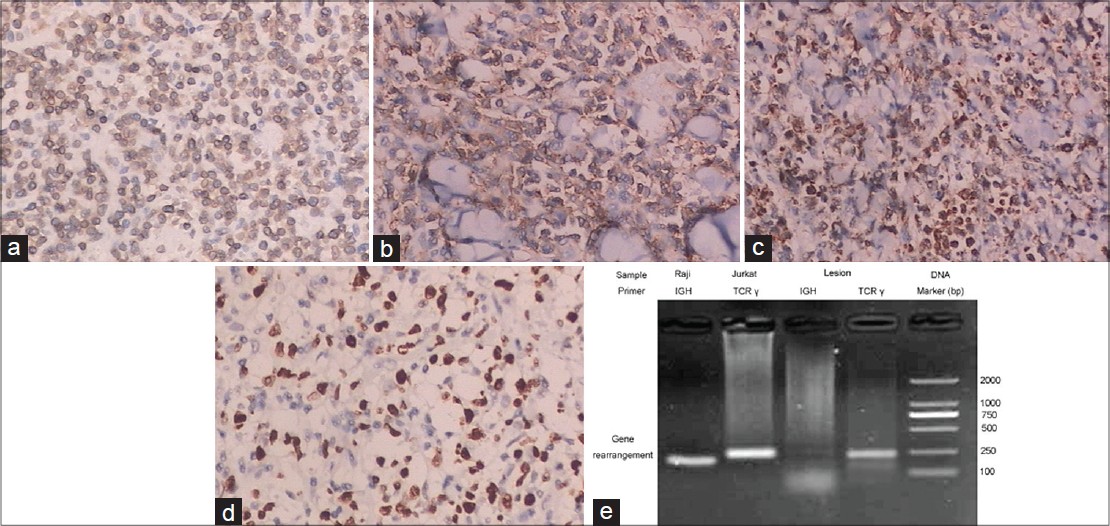 |
| Figure 2: Neoplastic cells in the biopsy of left arm are strongly positive for CD3 (a), CD56 (b), granzyme B (c), perforin and Ki-67(d). (e) Polymerase chain reaction (PCR) using a primer for the V and J regions of T-cell receptor (TCR) γ chain showed monoclonal rearrangement, indicating the presence of a monoclonal T-cell population |
After discussion with hematology and pathology colleagues, we made a diagnosis of peripheral T-cell lymphoma, not otherwise specified (NOS) as described in the 2008 World Health Organization (WHO) classification for cutaneous lymphoma. [4] Laboratory evaluation revealed pancytopenia [red blood cell count of 3.36 × 10 12 /L (4-5.5 × 10 12 /L), white blood cell count of 1.98 × 10 9 /L (4-10 × 10 9 /L), hemoglobin 90 g/L (120-160 g/L), platelet count 76 × 10 9 /L (100-300 × 10 9 /L), T-lymphocyte subtype, CD4 18% (40-55%), CD8 27% (18-30%), and CD4/CD8 0.67 (0.9-3.6).], hyperbilirubinemia [total bilirubin 24.8 μmol/L (2-18 μmol/L)], transaminitis [aspartate aminotransferase (AST) 223 U/L (<64 U/L), alanine aminotransferase (ALT) 283 U/L (<64 U/L) and raised lactic dehydrogenase 723 U/L (80-285 U/L) levels. A chest X-ray showed mildly increased pulmonary markings and a chest and abdomen computed tomographic (CT) scan showed mild bilateral pleural effusion, left axillary adenopathy, and splenomegaly. Examination of the oral cavity and pharynx was unremarkable. A bone marrow biopsy demonstrated dyshematopoiesis of myeloid cells and erythroid cells. The patient received 15 mg dexamethasone daily for high fever and liver-protective drugs for elevation of liver enzymes. But fever persisted and the tumor on his left arm enlarged in size to 8 × 10 cm in diameter with obvious necrosis. The patient was then transferred to the department of hematology. Combination chemotherapy with ifosfamide, pirarubicin, vindesine, and methylprednisolone were administered due to his severe systemic symptoms. Two days later, the laboratory data revealed progressively abnormal liver function tests (AST 667 U/L and ALT 1,060 U/L). The combination chemotherapy was changed to arsenic trioxide and dexamethasone. Although plaques on the trunk showed some improvement, the patient′s general condition continued to deteriorate as he developed progressive liver dysfunction and severe hyperbilirubinemia (total bilirubin 196.2 and direct bilirubin 179.3). A bone marrow biopsy at this time also showed infiltration with abnormal cells. The patient died on the 46 th hospital day.
DISCUSSION
Local or systemic vaccine reactions usually disappear spontaneously. [1] However, cases of vaccination-induced pseudolymphoma have been reported to occur after the administration of vaccines with aluminum oxide, an adjuvant added to prolong the period of activity of the vaccine. Maubec et al. [2] reported a series of nine patients presenting with cutaneous and subcutaneous pseudolymphomas. All of these lesions occurred at the site of anti-hepatitis B (eight cases) and anti-hepatitis A (one case) vaccination. Stavrianeas et al. and Cerroni et al. observed and reported similar cases. [3],[5] May et al. reported a patient who developed a low-grade cutaneous marginal zone B-cell lymphoma (MZL). [6] We were unable to find any reports of the development of a cytotoxic T cell lymphoma at injection site after searching Medline through PubMed.
In our patient, an ulcerated tumor developed initially at the site of vaccination, and then multiple red plaques appeared on his trunk and extremities. Immunohistochemistry and molecular analysis of the skin biopsies excluded extranodal NK/T-cell lymphoma, [7] primary cutaneous CD4-positive small/medium T-cell lymphoma (CD4 + SMTL), primary cutaneous CD8-positive aggressive epidermotropic T-cell lymphoma (CD8+ AECTCL), and primary cutaneous gamma/delta T-cell lymphoma (CGD-TCL). [8] The diagnosis of peripheral T-cell lymphoma, not otherwise specified (NOS) was established. The association of the lymphoma with the vaccination and the underlying mechanisms for the occurrence of T-cell lymphoma were unclear. Influenza virus vaccine (FLUARIX; ) administered to our patient has been standardized for the influenza season and is formulated to contain hemagglutinin, Triton X-100, and Tween 80. The vaccine does not contain preservatives and aluminum adjuvant. Most experts consider that chronic antigenic stimulation, local infection, inflammatory responses, and even the patient′s immune status might play an important role in the process of malignant transformation. Our patient′s clinical course was characterized by high fever, pancytopenia, splenomegaly, hyperferritinemia, and hemophagocytosis in the bone marrow; features suggesting that lymphoma-associated hemophagocytic syndrome (LAHS) should be considered as the cause of death. [9] In general, cutaneous T cell lymphomas expressing CD56, granzyme B, and perforin often exhibit a rapidly progressive course and poor response to chemotherapy. [10]
Acknowledgment
This work was supported by grants from Natural Science Foundation of Shanghai (No. 11ZR14474000), Special Fund for Military Medicine of SMMU (2012).
| 1. |
Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2010;375:56-66.
[Google Scholar]
|
| 2. |
Maubec E, Pinquier L, Viguier M, Caux F, Amsler E, Aractingi S, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol 2005;52:623-9.
[Google Scholar]
|
| 3. |
Cerroni L, Borroni RG, Massone C, Chott A, Kerl H. Cutaneous B-cell pseudolymphoma at the site of vaccination. Am J Dermatopathol 2007;29:538-42.
[Google Scholar]
|
| 4. |
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008.
[Google Scholar]
|
| 5. |
Stavrianeas NG, Katoulis AC, Kanelleas A, Hatziolou E, Georgala S. Papulonodular lichenoid and pseudolymphomatous reaction at the injection site of hepatitis B virus vaccination. Dermatology 2002;205:166-8.
[Google Scholar]
|
| 6. |
May SA, Netto G, Domiati-Saad R, Kasper C. Cutaneous lymphoid hyperplasia and marginal zone B-cell lymphoma following vaccination. J Am Acad Dermatol 2005;53:512-6.
[Google Scholar]
|
| 7. |
Gniadecki R, Rossen K, Ralfkier E, Thomsen K, Skovgaard GL, Jønsson V. CD56+lymphoma with skin involvement. Clinicopathologic features and classification. Arch Dermatol 2004;140:427-36.
[Google Scholar]
|
| 8. |
Kempf W, Rozati S, Kerl K, French LE, Dummer R. Cutaneous peripheral T-cell lymphomas, unspecified/NOS and rare subtypes: A heterogeneous group of challenging cutaneous lymphomas. G Ital Dermatol Venereol 2012;147:553-62.
[Google Scholar]
|
| 9. |
Blom A, Beylot-Barry M, D'Incan M, Laroche L. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol 2011;65:404-10.
[Google Scholar]
|
| 10. |
Zindanci I, Kavala M, Buyukbabani N, Kocaturk E, Koc M. Cutaneous CD4+/CD56+hematodermic neoplasm. Indian J Dermatol Venereol Leprol 2010;76:723.
[Google Scholar]
|
Fulltext Views
3,284
PDF downloads
1,651





