Translate this page into:
Persistent pruritic rash: A rare manifestation and possible poor prognostic sign in adult onset Still's disease
2 Department of General Medicine, Govt Medical College, Kozhikode, Kerala, India
3 Department of Pathology, Govt Medical College, Kozhikode, Kerala, India
Correspondence Address:
Sarita Sasidharanpillai
'Rohini', Girish Nagar, Nallalam PO, Kozhikode 27, Kerala
India
| How to cite this article: Riyaz N, Manikath N, Sasidharanpillai S, Govindan A, Davul H, Rini MR. Persistent pruritic rash: A rare manifestation and possible poor prognostic sign in adult onset Still's disease. Indian J Dermatol Venereol Leprol 2015;81:548 |
Sir,
Adult-onset Still′s disease is a rare inflammatory disorder characterized by high spiking fever, polyarthralgia/arthritis, characteristic evanescent, salmon pink rash (that fades when the fever remits and reappears with the next spike) and multi-organ involvement. Typical Still′s rash is difficult to identify in dark-skinned races. [1] The presence of persistent pruritic plaques and linear pigmentation that do not change with febrile variations, reported occasionally in adult-onset Still′s disease is suggested to be a bad prognostic sign. [2] We report a patient of adult-onset Still′s disease with persistent itchy rash who developed fatal pulmonary embolism.
A 22-year-old woman was admitted in the general medicine department of Government Medical College, Kozhikode with a two-week history of intermittent, evening-rise fever and arthralgia accompanied by persistent, extremely itchy skin lesions. She had also had a sore throat which began a week after the onset of her other symptoms. Non-steroidal anti-inflammatory drugs (NSAIDs) initiated 5 days after the onset of symptoms produced no improvement.
At presentation, she was febrile (42°C) with pain and tenderness of the wrist, knee and ankle joints. The ankle joints showed edema, erythema [Figure - 1] and restriction of movements due to pain. Her forehead and anterior neck showed discrete and confluent, erythematous to violaceous, scaly papules and plaques with crusting and koebnerization [Figure - 2]a. On her anterior abdomen, lower back and groins, she had violaceous linear lesions suggestive of flagellate dermatitis [Figure - 2]b. [3] Her skin lesions were intensely pruritic without pain, tenderness, or scarring. Systemic examination was within normal limits without any evidence of proximal muscle tenderness or weakness.
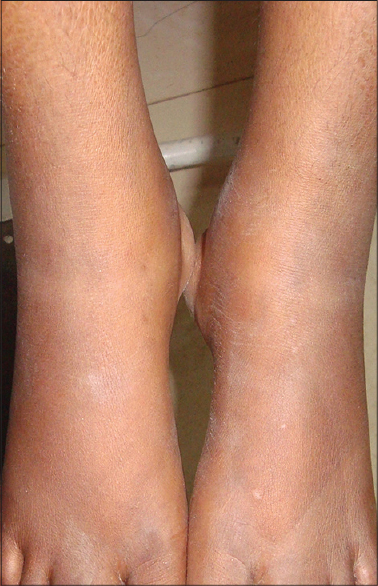 |
| Figure 1: Erythema and edema of ankle joints |
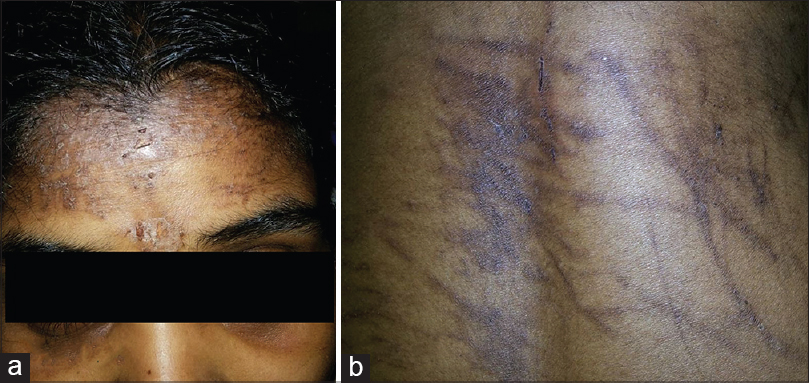 |
| Figure 2: (a) Violaceous erythematous scaly rash with crusting on the forehead. (b) Flagellate dermatitis on the back of trunk |
Investigations revealed neutrophilic leukocytosis (total count 24,000 cells/mm 3 , 91% neutrophils), thrombocytosis (6.8 lakh cells/mm 3 ) and elevated erythrocyte sedimentation rate (120mm/h) and C-reactive protein (9.6 mg%). A peripheral smear also showed neutrophilia and thrombocytosis. Serum lactate dehydrogenase (2772 U/l), aspartate aminotransferase (143 IU/ml) and serum ferritin (1500 ng/ml) levels were elevated whereas antinuclear antibody profile and rheumatoid factor were negative. Blood and urine cultures were sterile. Serological investigations for hepatitis B, C and A viruses, typhoid, dengue, chikungunya, leptospira and rickettsia were all negative. Chest radiography, echocardiogram, ultrasonogram of abdomen and pelvis, and electromyogram detected no abnormality. A skin biopsy taken from the linear lesion on the back showed a small focus of basal cell degeneration and prominent perivascular infiltration by mixed inflammatory cells (mainly neutrophils) with nuclear dust without any evidence of vasculitis [Figure - 3]. The inflammatory infiltrate was mainly confined to the upper dermis.
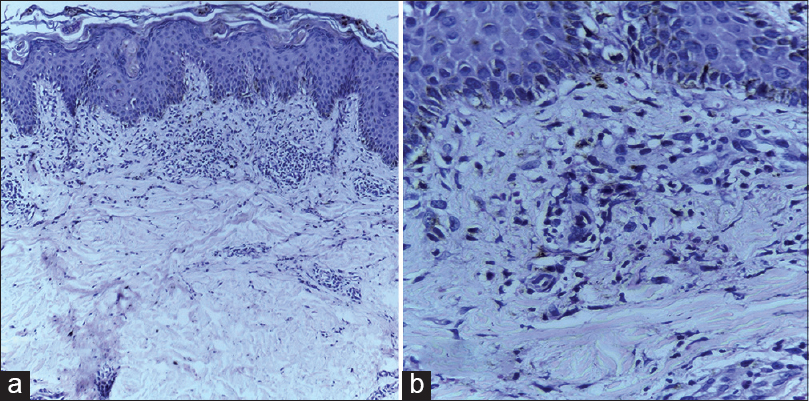 |
| Figure 3: (a) Biopsy from the lesion of flagellate dermatitis on the back showing a mixed inflammatory infiltrate in the upper dermis (H and E, ×100). (b) The inflammatory infiltrate is composed predominantly of neutrophils and nuclear dust around vessels and is accompanied by pigment incontinence (H and E, ×400) |
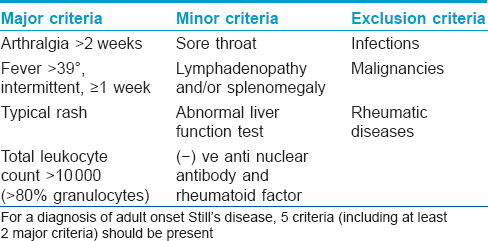
Since investigations ruled out most infective, autoimmune and neoplastic entities, and as the patient satisfied the Yamaguchi criteria [Table - 1], a final diagnosis of adult onset Still′s disease was made. [4]
She was treated with methyl prednisolone pulse therapy, 500 mg in 500ml of 5% dextrose for 3 days, followed by prednisolone, 50 mg daily and azathioprine, 100 mg in two divided doses daily. Fever and joint symptoms improved markedly with pulse therapy but worsened soon after. Erythrocyte sedimentation rate and C-reactive protein levels also showed a rise following an initial decline during pulse therapy. A re-evaluation ruled out macrophage activation syndrome and infective causes. There was progressive worsening of symptoms over the two days following pulse therapy. On the third post-pulse day, the patient developed acute pulmonary embolism and expired before thrombolytic treatment could be initiated.
The differential diagnosis for persistent skin lesions of adult-onset Still′s disease includes lichen planus, lupus erythematosus, dermatomyositis and lichenoid drug eruption.
Features that strongly suggested that our patient had adult-onset Still′s disease-associated rash included the morphological presentation of papules and plaques along with bizarre linear lesions, a neutrophil-predominant dermal infiltrate on histology, and the setting of intermittent high grade fever, arthralgia, neutrophilic leukocytosis, elevated serum ferritin, negative ANA profile and rheumatoid factor and normal electromyogram.The combination of necrotic keratinocytes in the upper epidermis and neutrophil-predominant dermal infiltrate are cited as the histological features that differentiate the persistent eruption of adult-onset Still′s disease from most other interface dermatitides including lupus erythematosus, where the necrotic keratinocytes are noted in the lower half of the epidermis and dermis with infiltration of lymphocytes in the basal portion of the epidermis. [2] Whether a biopsy from the violaceous scaly plaques in our patient (instead of the linear lesion) would have revealed keratinocyte necrosis remains unknown. Basal vacuolar alteration and nuclear dust have been noted occasionally in the persistent rash of adult-onset Still′s disease. [2]
Drug-induced lichenoid eruption was unlikely in our patient, since the appearance of rash preceded all drug intake and the dermal infiltrate lacked eosinophils, the common inflammatory cell in drug eruptions. [2]
Lee et al. reported 11 cases of adult-onset Still′s disease who manifested a non-evanescent, persistent pruritic rash with bizarre linear lesions in some (attributed to koebnerization due to scratching), [2] which were very similar to the findings in our patient. Persistent Still′s rash is attributed to elevated serum levels of tumor necrosis factor-α, interferon-γ and interleukin-8 with up regulation of Fas ligand on interferon-γ stimulated keratinocytes, thereby inducing keratinocyte necrosis. [2]
Treatment options in adult-onset Still′s disease include NSAIDs, systemic steroids and immunosuppressants (methotrexate, azathioprine, cyclosporine). [5] The rare, steroid unresponsive adult-onset Still′s disease (like our case) may benefit from intravenous immunoglobulin G (IVIG) or biological agents such as infliximab, etanercept and anakinra. [5],[6]
Acute pulmonary embolism manifested by our patient is a rare, life-threatening complication of adult-onset Still′s disease. [7] Lee et al. have noted that adult-onset Still′s disease associated with persistent rash has a poor prognosis. [2] The development of fatal pulmonary embolism in our patient underscores the need for more data on the prognostic significance of persistent rash in adult-onset Still′s disease. Whether intravenous immunoglobulin or biologicals would have reversed the course of the disease in our case remains unknown. We recommend close monitoring and aggressive treatment of patients manifesting adult-onset Still′s disease with persistent rash.
| 1. |
Chandran V, Aggarwal A, Misra R.Adult-onset still′s disease. J Indian Rheumatol Assoc 2002:10:19-21.
[Google Scholar]
|
| 2. |
Lee JY, Yang C, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still′s disease. J Am Acad Dermatol 2005;52:1003-8.
[Google Scholar]
|
| 3. |
Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol 2014;80:149-52.
[Google Scholar]
|
| 4. |
Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still′s disease. J Rheumatol 1992;19:424-30.
[Google Scholar]
|
| 5. |
Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset still′s disease. Ann Rheum Dis 2006;65:564-72.
[Google Scholar]
|
| 6. |
Maier J, Birkenfeld G, Pfirstinger J, Schölmerich J, Fleck M, Brühl H. Effective Treatment of Steroid Refractory Adult-Onset Still′s Disease with Anakinra.J Rheumatol 2008;35:939-41.
[Google Scholar]
|
| 7. |
Al-Temimi FA, George P. Adult Onset Still′s Disease in Oman. Sultan Qaboos Univ Med J 2006;6:41-5.
[Google Scholar]
|
Fulltext Views
3,001
PDF downloads
3,670





