Translate this page into:
Phenomena in dermatology
2 Department of Dermatology, NKP Salve Institute of Medical Sciences and Lata Mangeshkar Hospital, Hingna, Nagpur, India
Correspondence Address:
Uday Khopkar
Department of Dermatology, Seth GS Medical College and KEM Hospital, Mumbai - 400 012
India
| How to cite this article: Madke B, Doshi B, Pande S, Khopkar U. Phenomena in dermatology. Indian J Dermatol Venereol Leprol 2011;77:264-275 |
Abstract
For a better understanding of various dermatoses, it is imperative for any physician practising dermatology to have a good theoretical knowledge of the underlying pathophysiologic processes involved in various systemic diseases involving the skin. For an easy grasp over this topic, we have discussed the various phenomena under three broad categories, like (a) clinical - Meyerson, Meirowsky, pathergy, Renbok, (b) laboratory - LE cell, prozone and (c) histopathology - Splendore-Hoeppli."To study the phenomenon of disease without books is to sail an uncharted sea, while to study books without patients is not to go to sea at all."
Sir William Osler-
British (Canadian born) physician (1849-1919)
Introduction
Phenomenon is defined as any sign or objective symptom; any observable occurrence or fact. [1] In the dermatological literature, the term phenomenon has been applied to several peculiar clinical occurrences or laboratory observations. In this article, an attempt will be made to describe the phenomenon relevant to dermatologists and will be discussed under three categories, i.e. clinical, laboratory and histopathology. Having a thorough knowledge of these phenomena will help us to better understand the disease process and give our patients a rational and realistic care.
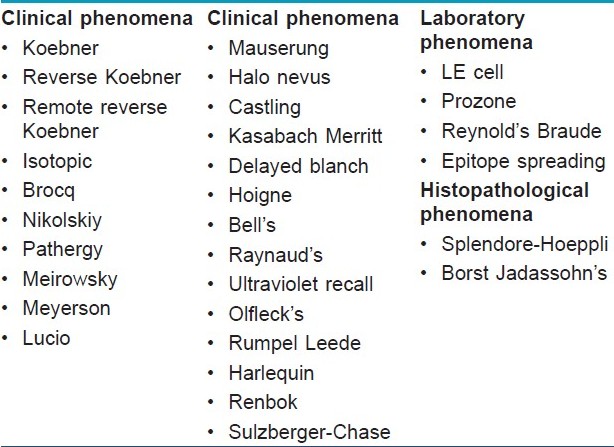
Phenomena in dermato-venereology can be divided into three broad categories, those described in a clinical setting, in the laboratory or as a histopathological observation [Table - 1].
Clinical Phenomena
Koebner/Isomorphic phenomenon
In 1877, Heinrich Koebner described the appearance of psoriatic lesions in the uninvolved skin of psoriatic patients as a consequence of trauma. [2] This phenomenon has been dealt in greater detail by several authors and hence will not be further discussed. [3],[4],[5] In general, the interval to koebnerization is between 10 and 20 days, but may be as short as 3 days or as long as 2 years. [6] The original classification of Koebner phenomenon has been given in [Table - 2]. The purpose of this article is to bring to the fore some of the less well-known phenomena.

Reverse Koebner phenomenon
This phenomenon is explained, in which an area of psoriasis clears after injury, like dermabrasion or surgery. [6]
Remote reverse Koebner phenomenon
It is defined in vitiligo patients, in which spontaneous repigmentation is seen in distant patches after autologous skin graft surgery. [6]
Isotopic phenomenon (Wolf′s isotopic response)
Isotopic phenomenon is best described in herpes zoster. Occurrence of a different or unrelated dermatological disease at the site of the healed disease (commonly herpes zoster) is termed as isotopic phenomenon (iso - same, topic - site) [7] The exact cause of this phenomenon is not known. The proposed etiologies of isotopic response are viral, immunologic, neural, vascular and locus minoris resistentiae (a site of lessened resistance). Virus-induced local neuroimmune dysregulation of the dermal sensory nerve fibers has been suggested as a possible explanation. [7] Most cases of isotopic response have been described in healed lesions (scars) of herpes zoster. The second unrelated disease has been reported to be granuloma annulare, Kaposi′s sarcoma, leukemia cutis, metastasis, sarcoidosis, acne, lichen planus, [8] granulomatous folliculitis, [9] tinea, verruca plana, molluscum contagiosum, squamous cell carcinoma, basal cell carcinoma or multiple epidermoid cysts. [10]
Brocq′s phenomenon
Brocq′s phenomenon refers to subepidermal hemorrhage, which occurs on careful scraping of a classical lesion of lichen planus. This is in contrast to the scratching of the surface of lesions of psoriasis, which results in pin-point bleeding. [11]
Nikolskiy′s phenomenon [12]This phenomenon was described by Sir Wilhelm Lutz. The term "Nikolskiy phenomenon" is applied when the superficial layer of the epidermis is felt to move over the deeper layer, and instead of immediately forming erosion as in Nikolskiy′s sign, a blister develops after some time. This phenomenon is seen in vesiculobullous disorders that show a positive Nikolskiy sign, such as pemphigus vulgaris.
The significance of this phenomenon is that it indicates a lower disease activity in patients of pemphigus vulgaris than that of patients who have a positive Nikolskiy sign.
Pathergy phenomenon [13]Pathergy phenomenon is most commonly described in pyoderma gangrenosum and Behcet′s disease, and is also noted in some cases of sweet′s syndrome and subcorneal pustular dermatosis. Heavy neutrophlic infiltrates or pustular reaction that develop at the site of non-specific trauma are termed as pathergy phenomenon. Pathergy phenomenon is tested by the pathergy test, and it refers to the development of a new lesion at the site of eliciting the test. An erythematous papule of more than 2 mm at the prick site with a 20-22-gauge needle (to avascular skin obliquely to the depth of 5 mm following an intradermal injection of 0.1 ml of normal saline) is called as a positive pathergy test. Positive pathergy is regarded as one of the minor criteria for the diagnosis of Behcet′s disease by the International Study Group Diagnostic criteria for Behcet′s disease.
Meirowsky phenomenon
Meirowsky in 1909 discovered that pieces of fresh human skin showed irreversible darkening of the epidermal melanin if heated. [14] This type of heat darkening came to be known as the Meirowsky phenomenon. Meirowsky phenomenon is among the first responses of human skin to ultraviolet (UV) exposure. It is also called as immediate pigment darkening (IPD). IPD is more pronounced within a few hours of sun exposure, and is due to metabolic changes coupled with the redistribution of melanin already present in the skin. The phenomenon is seen after exposure to long-wave UVB, UVA and, to some extent, visible radiation. IPD is not photo protective as it does not lead to a hardening effect as seen in delayed tanning. [15]
Meyerson phenomenon [Figure - 1]
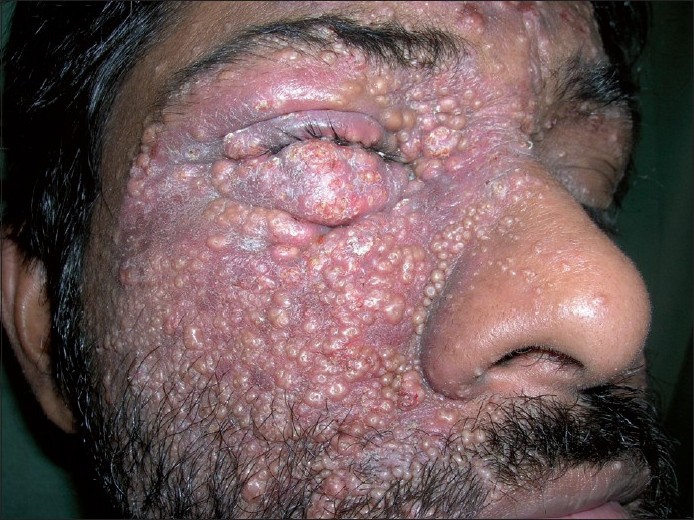 |
| Figure 1: Meyerson phenomenon: exuberant eczematization seen around numerous molluscum contagiosum |
The phenomenon was described by Meyerson in 1971 [16] in a patient who presented with eczematous reaction around a congenital melanocytic nevus. Later on, such type of a nevus presenting with eczematous changes around it was termed as Meyerson′s nevus.
The Meyerson phenomenon is also known as halo dermatitis or halo eczema, which should not be confused with halo nevus or Sutton′s nevus, which is a melanocytic nevus surrounded by either hypo or depigmentation. However, progression of Meyerson′s nevus into Sutton′s nevus has been reported. [17] Meyerson′s phenomenon has been noticed in seborrheic keratosis, [18] stucco keratosis, molluscum contagiosum, [19] dermatofibromas, basal cell, squamous cell carcinomas [20] and in dysplastic nevus after interferon alfa-2b therapy. [21]
The exact mechanism for such type of a perilesional eczematous eruption is not known; it is hypothesized to be an immune-mediated reaction. Histopathology is consistent with spongiotic dermatitis with dermal infiltrate of eosinophils. However, the condition responds to topical steroids or removal of the initiating lesion.
Lucio phenomenon
Lucio phenomenon or erythema necroticans (not necrotic erythema nodosum leprosum) was named after Lucio and Alvarado after they published a monograph on leprosy in 1852. [22] It is a very peculiar type of reactional state, characterized by distinct clinical and histopathological features that classically occur in patients with undiagnosed and untreated, non-nodular, diffuse, lepromatous leprosy, known as Lucio′s leprosy, or in untreated pure primitive diffuse lepromatous leprosy. [23]
Necrotic, irregular, geometric ulcers are characteristic of this reactional state. [24] The evolution of lesions is very typical, progressing from erythematous, ill-defined and painful plaques to jagged-edged skin ulcerations. The lesions are extensive and distributed over the legs, thighs, forearms and buttocks. In this condition, the lepromin test gives an intense local response (4-6 h), the Medina-Ramirez reaction. [25] It may clearly imitate or simulate Lucio′s reaction. This exaggerated lepromin reaction is partly explained by the Schwartzman type of bacterial hypersensitivity.
Histopathological findings that are peculiar to this condition include colonization of the endothelial cells by acid fast bacilli, ischemic epidermal necrosis, necrotizing vasculitis of the small vessels of the superficial dermis, endothelial proliferation of the medium-sized vessels of the mid-dermis with passive venous congestion and neutrophilic infiltration. [26]
The World Health Organization (WHO) multidrug therapy (MDT) is also reported to be effective in the treatment of Lucio′s phenomenon. However, concerns have been raised regarding the WHO MDT, as it could lead to more damage by increasing the quantity of the immune complexes after bacterial killing. [27]
Mauserung phenomenon [28]
In 1937, Siemens reported a family with features similar to, but distinct from, bullous congenital ichthyosiform erythroderma of Brocq (BCIE). This variant has been termed ichthyosis bullosa of Siemens (IBS), and has been reported to be distinguished clinically from BCIE by the absence of erythroderma, localization of dark grey hyperkeratosis to the flexural sites and areas of peeling of the skin among hyperkeratotic areas known as the "mauserung" phenomenon.
A distinctive characteristic of IBS, which is not present in the other forms of ichthyosis, is called the "mauserung" phenomenon (mauserung is German for "moulting"). These are small patches of bare, apparently normal skin (due to regeneration of the epidermis) in the middle of areas of hyperkeratosis.
Halo phenomenon [Figure - 2]
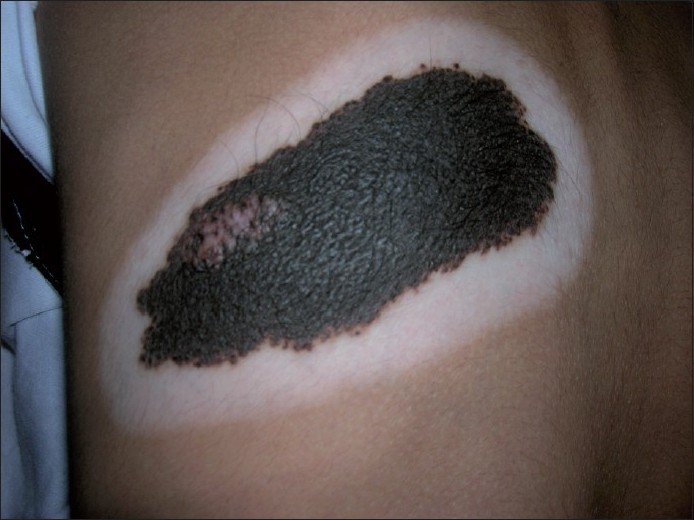 |
| Figure 2: Halo phenomenon: destruction of melanocytes around a congenital melanocytic nevus resulting in a depigmented halo |
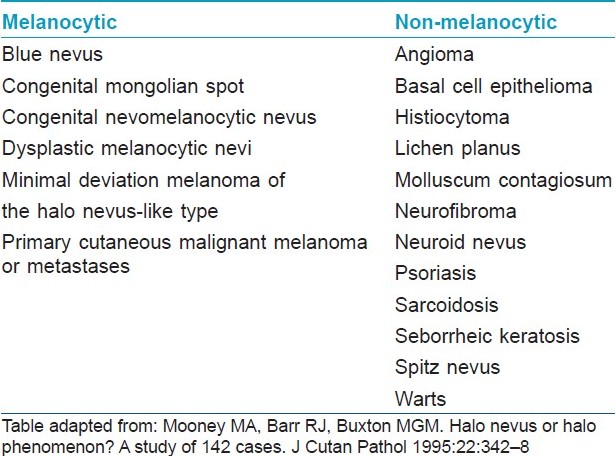
The halo phenomenon has been described in various melanocytic and non-melanocytic neoplasms as well as in non-neoplastic conditions [29] [Table - 3], but it is classically seen in halo nevus or Sutton′s nevus or perinevoid vitiligo. Halo nevus was originally described by Hebra and popularized by Sutton as leukoderma acquisitum centrifugum in 1916. [30]
The etiology of the halo phenomenon is unclear. The microscopic criteria for the halo phenomenon include the obligate presence of a band-like lymphohistiocytic infiltrate and a diminution or absence of melanin pigment at the dermoepidermal junction at the periphery of the nevus. It seems to occur either following exposure to actinic radiation, external depigmenting agents or, most often, idiopathically. [31] The typical history of halo nevus phenomenon is that a ring of depigmentation starts around a pre-existing pigmented lesion.
Castling phenomenon
This term is derived from repositioning of the king with the rook in chess, describing the rare paradoxical hair growth observed on the untreated scalp side in patients receiving topical contact sensitizer treatment on the contralateral side. [32] The castling phenomenon was originally described by Happle et al. as "rochade," or "castling." [7] It has also been referred to as "crossing." In most patients, hair grows at the application site, but regrowth at distant sites (face, eyebrows, eyelashes, body, axillae, pubis) may also occur. [33]
Such paradoxical growth has been hypothesized to be caused by the distinct thresholds of different skin areas to treatment. [34] Others have implicated a role for the nervous system and neurotransmitters. A unifying theory to explain such paradoxical local and systemic effects is still lacking. The mechanism is unclear, but a systemic effect of localized application has been proposed. It has been observed in 1-2% of the cases after topical application of squaric acid dibutyl ester or diphencyprone.
Kasabach Merritt phenomenon
The Kasabach Merritt phenomenon is a triad comprising of vascular tumors, thrombocytopenia and bleeding diathesis. This triad was described by Kasabach and Merritt in 1940. [35] But, when a similar clinical picture was noted in other vascular tumors, the term Kasabach Merritt phenomenon was coined [Table - 4].

The pathogenesis of Kasabach Merritt phenomenon is presumed to be due to trapping of platelets in the abnormal vasculature in the tumor as confirmed by studies with Indium 111 (I111)-labeled platelets, [36],[37] which subsequently leads to activation of platelets and consumption of clotting factors. Thrombocytopenia is usually profound with counts <20 X 10 9 /L (<20,000/mm 3 ), and also the platelet half-life is drastically reduced from normal of 7 days to between 1 and 24 h. [38]
This continued consumption of platelets and clotting factors along with initiation of fibrinolysis eventually results in intralesional bleeding, which manifests as rapid enlargement of the hemangioma and, therefore, the cycle continues.
Laboratory evidence of the consumptive coagulopathy includes decreased platelets, fibrinogen and factors II, V and VIII: increased prothrombin time, partial thromboplastin time and fibrin-split products; and microangiopathic hemolytic anemia.
Delayed blanch phenomenon [40]This phenomenon is characteristically seen in atopic individuals. It is based on the fact that atopic patients have an enhanced tendency of the skin to undergo vasoconstriction. This phenomenon is seen in all the individuals who have inherited the trait, irrespective of whether they have clinical features of atopic disease or not.
It is demonstrated by injecting 0.1 ml (0.025 mg) of carbachol or pilocarpine intradermally into the volar aspect of the forearm. In a non-atopic individual, this leads to a wheal and erythema, which lasts for a variable period of time. On the other hand, in atopic patients, after a period of 3-5 min, the wheal becomes pale and this pallor (blanch) spreads beyond the wheal, into the surrounding area of the erythema The test is considered positive only if this blanch spreads beyond the wheal.
Hoigne′s phenomenon (Procaine psychosis)
Psychotic symptoms arising as a complication of treatment with procaine penicillin with classic features of anxiety/panic and hallucinations have been termed Hoigne′s phenomenon. Accidental intravascular injection of procaine penicillin results in mental confusion, visual and auditory hallucination, sense of impending doom, headache and hypotension. This symptom complex occurs due to the lipid-soluble procaine moiety, which readily crosses the blood-brain barrier. In dermatology, it was observed in cases of primary and secondary syphilis who had been treated with procaine penicillin. [41]
At present, there are two theories regarding the cause of non-allergic procaine penicillin reactions. The vascular theory was first proposed by Batchelor et al. [42] Hoigne too proposed micro-embolization of the small vessels in the lungs and brain by microcrystals of procaine penicillin dislodged from the site of injection. The toxic theory proposes that the reaction is caused by the toxic action of procaine on the central nervous system. [43]
Bell′s phenomenon [Figure - 3]
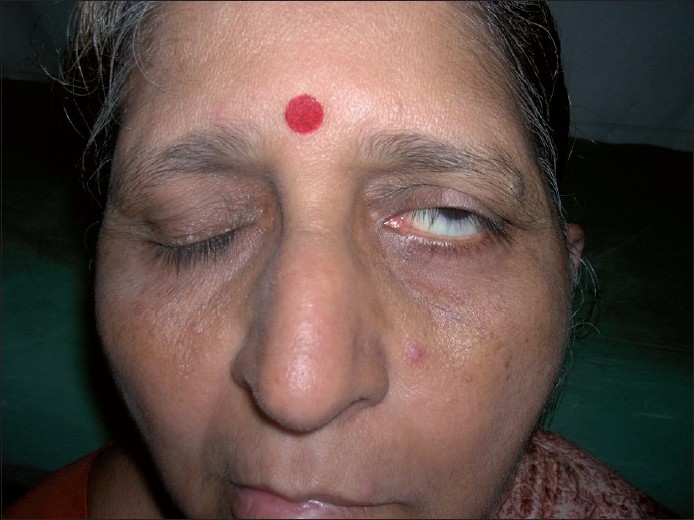 |
| Figure 3: Bell's phenomenon: inability to close the affected eye, resulting in visibility of the lower portion of the sclera |
The phenomenon is named after the Scottish anatomist, surgeon and physiologist, Charles Bell. [44] Bell′s phenomenon is classically described in Bell′s palsy. Bell′s palsy is idiopathic lower motor neuron type of seventh cranial nerve paralysis. Normally, before retiring to sleep, both the eye balls turn upwards as a protective mechanism to prevent injury to the cornea, and this is not evident clinically.
The phenomenon is elicited by asking the patient to forcibly close both his eyes. On attempted closure of the affected side, there is up-rolling of the eye ball, and this is clinically seen as the patient is not able to close his affected eye. Also, this phenomenon helps to make a distinction between the upper and the lower motor neuron type of facial nerve paralysis, and helps the clinician to locate the site of lesion.
In dermatology, the lower motor neuron type of seventh nerve paralysis is seen in cases of Melkerson Rosenthal syndrome (unilateral or bilateral affection of facial nerve, fissured tongue and granulomatous cheilitis), herpes zoster oticus (Ramsay Hunt syndrome type II) and Hansen′s disease. However, Bell′s phenomenon is not considered pathognomonic for facial nerve palsy as it is present in 75% of the normal individuals, [45] and should be interpreted with other clinical signs suggestive of seventh nerve palsy.
Raynaud′s phenomenon
It is a well-known phenomenon in internal medicine as well as in dermatology, first described by the French physician Maurice Raynaud in 1862, although the term was coined later by Hutchinson. [46] It is a vasospastic disorder characterized by episodic changes in blood flow in the cutaneous vasculature. Raynaud′s phenomenon is seen in the colder season and is clinically characterized by triphasic color change of pallor, cyanosis and hyperemia. In the first phase, due to intense vasospasm, the skin of the digits turns pale to dead white in color as a result of decreased blood flow. The next phase involves bluish-black discoloration of skin due to venous congestion and increased concentration of deoxygenated blood in the peripheral circulation. Both the above episodes are associated with intense pain due to cutaneous ischemia. On rewarming, there is rubor and erythema of the digits due to vasodilatation and accumulation of vasodilator peptide-like substance P and prostaglandins during the initial two phases. This third phase can lead to reperfusion injury due to production of reactive oxygen species analogous to reperfusion injury in myocardial infarction.
Repeated episodes of Raynaud′s phenomenon can lead to digital infarction, necrosis and resorption of the terminal phalanx, which is clinically evident as pitted scars and digital shortening. The phenomenon can be elicited by an ice-cube test, in which an ice-cube wrapped in gauze piece is placed in the patient′s hand for a period 20 min and color changes, if any, are noted. The causes of Raynaud′s phenomenon have been broadly divided into idiopathic and secondary causes [Table - 5].

Ultraviolet recall phenomenon
Syn: Erythema recall, photo recall, radiation recall.
There are two types of photo recall phenomena of interest to the dermatologist: radiation and ultraviolet recall. It is an acute inflammatory reaction within a previously irradiated or sunburned area after the administration of systemic chemotherapeutic agents like methotrexate or cytarabine. [48] Most of the cases of ultraviolet recall have been reported in psoriatics with the use of methotrexate immediately following PUVA or UVB therapy. Hence, methotrexate should be introduced after 2 weeks of stopping of any form of phototherapy. The exact mechanism for recall phenomenon remains speculative. It has been suggested that the photo recall phenomenon (ultraviolet recall phenomenon) may represent a form of Koebner phenomenon. [49]
Olfleck′s phenomenon
In psoriatic nail involvement, there is brownish discoloration of the nails. This brownish coloration of the nails is due to subungal hyperkeratoses, which occur due to the collection of parakeratotic columns in the nail bed, and is termed as "Olfleck′s" phenomenon. [50]
Rumpel Leede phenomenon
Rumpel Leede phenomenon, or acute dermal capillary rupture, is classically seen in microvascular fragility disorders. It is said to be present when there are more than 20 petechiae/square inch after inflating the blood pressure cuff to a point between systolic and diastolic blood pressure for 5 min. [51] The Rumpel Leede phenomenon is described in conditions like scurvy, [51] bleeding disorders such as dengue fever or thrombocytopenia and drug-induced erythema multiforme. [52]
Harlequin phenomenon
The harlequin color change is an unusual cutaneous phenomenon observed in newborn infants as transient, benign episodes of a sharply demarcated erythema on one half of the infant, with simultaneous contralateral blanching. [53] The characteristic abnormality is a migratory pink color involving one lateral half of the infant′s body, with simultaneous pallor of the other half. The most common time for its appearance is between the second and fifth days of life, but it may occur as late as the third week of life. [54] The color changes develop suddenly, without obvious precipitating factors, and persist from 30 s to 20 min or more.
Renbok phenomenon (Inverse Koebner)
Renbok phenomenon was described in a patient suffering from two autoimmune diseases, i.e. psoriasis and alopecia universalis. [55] It is a well-known fact that psoriasis is a T-cell-mediated disorder, while alopecia universalis is a B-cell-mediated condition. In this patient, it was observed that psoriatic plaques on the scalp retained hair follicles, whereas hair loss was evident elsewhere. Also, it was noticed that scalp alopecia responded to contact sensitization with squaric acid dibutyl ester (SADBE). This phenomenon represented a natural experiment in which three distinct but overlapping immune responses favored psoriasis or contact dermatitis, thereby halting the progress of alopecia areata. [56]
Sulzberger-Chase phenomenon
Abolition of dermal contact hypersensitivity to sensitizing agents, e.g. picryl chloride, is produced by prior oral feeding of the agent. [57] In this form of desensitization, the patient is slowly exposed to a level of allergen that produces low-grade non-life-threatening anaphylaxis. At the end of the procedure, the patient′s mast cells have depleted their granular contents and the patient cannot undergo any allergic response until these cells restore the contents. [58]
Rebound phenomenon
In medicine, rebound refers to reappearance of the sign and symptom complex after stopping treatment, especially drugs; e.g. accelerated rise in blood pressure after sudden stoppage of anti-hypertensive therapy. However, in dermatology, rebound phenomenon is seen while the patient is using topical steroids. Topical steroids are routinely prescribed by the dermatologist for the treatment of dermatosis, like seborrheic dermatitis and scalp psoriasis. Likely explanation for rebound phenomenon can be downregulation of the cellular receptors, thereby decreasing the efficacy of topical steroids and flaring up of the underlying dermatosis. Tachyphylaxis, defined as a rapidly decreasing response to a physiologically active agent after administration of a few doses, can also be a plausible reason for rebound phenomenon. [59]
Histological Phenomena
Splendore-Hoeppli phenomenon [Figure - 4]
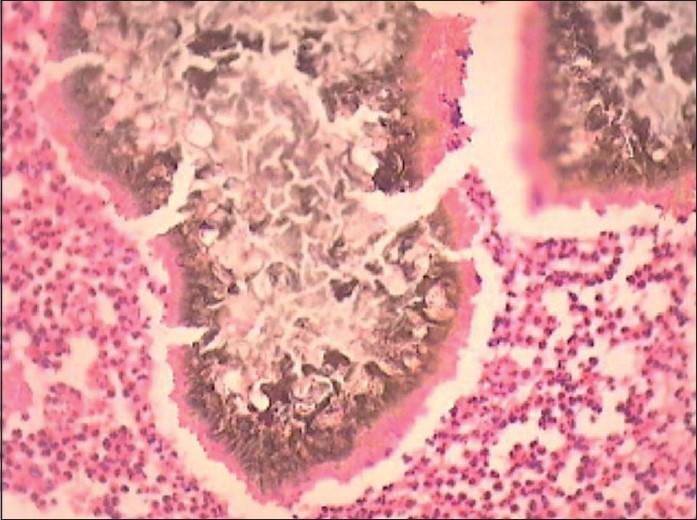 |
| Figure 4: Splendore Hoeppli phenomenon: brownish grains of actinomycetoma surrounded by an eosinophilic sleeve of immune complexes (H and E, ×200) |
The Splendore-Hoeppli phenomenon is a histopathological feature characterized by intensely eosinophilic material (radiate, star-like, asteroid or club-shaped configurations) around the microorganisms (fungi, bacteria and parasites) or biologically inert substances. This morphologically distinct reaction was first described by Splendore in sporotrichosis and Hoeppli in schistosomiasis. [60] The intensely eosinophilic material seen in the Splendore-Hoeppli reaction may represent the deposition of antigen-antibody complexes (immunoglobulins and major basic proteins) and debris from the host inflammatory cells [Table - 6].
Borst Jadassohn phenomenon
In 1904, Borst first observed nests of malignant cells at the edges of carcinoma of the lip, which were sharply defined from the surrounding hyperplastic epidermis. He interpreted these nests as being a secondary intraepidermal growth of the carcinoma. Epidermal nests occurring in the absence of dermal invasion were subsequently described by Jadassohn in 1926, who thought that these were intraepidermal basal cell carcinomas. However, Smith and Coburn proposed that the typical Jadassohn lesion should be regarded as of sweat gland origin, and the name of hidroacanthoma simplex was coined as it was characterized by well-demarcated nests of uniform, basaloid cells confined within the epidermis, which consistently contained large amounts of glycogen and stained positively with PAS, suggesting differentiation towards eccrine ducts. [62]
Mehregan and Pinkus concluded that Borst and Jadassohn had described quite different biological phenomena, and that the term "Borst-Jadassohn intraepidermal epithelioma" therefore should be discarded. They believed that Borst was describing a squamous cell carcinoma of the lip that secondarily invaded the epidermis, and that the Jadassohn lesion has a mixed etiology and that intraepidermal basal cell carcinoma seldom occurs. They found the Jadassohn phenomenon was most often associated with benign lesions such as seborrheic keratosis (especially if irritated by infection or trauma) or entirely intraepidermal sweat gland tumors (hidroacanthoma simplex of Smith and Coburn). [63]
Less commonly, it may be seen in pre-malignant or malignant conditions such as actinic keratosis, Bowen′s disease, melanoma in situ, Paget′s disease and epidermotropic metastatic carcinomas. [63]
Pagetoid phenomenon
Pagetoid is a term used in dermatology to refer to "upward spreading" of melanocytes into the epidermis. A pagetoid growth pattern with upward growth of the melanocytes, such that they are no longer confined to the basal layer, is considered pathognomonic for melanoma. Pagetoid phenomenon has also been noted in apocrine gland carcinoma, wherein it was demonstrated that the neoplastic cells arising from the apocrine gland were showing an intraepidermal Pagetoid pattern. [64]
Laboratory Phenomena
LE cell phenomenon
LE cell phenomenon was first described by Hargraves et al. [65] LE cells appear in the blood smear of patients of systemic lupus erythematosus. This phenomenon appears due to the action on leukocytic nuclei by autoantibodies (serum factor, LE cell factor) present in patient serum. These altered nuclei are extruded from the affected cells, which are then phagocytosed by viable leukocytes. LE cells are polymorphonuclear leukocytes (neutrophils, polymorphs) that have ingested nuclear material from the degenerated white cells. Three ingredients are necessary for the LE cell phenomenon to take place, which include serum factor (autoantibodies, previously called as LE cell factor), nuclei to be altered and leukocytes capable of phagocytosis. [66] About 5cc of heparinized blood is added to a conical flask containing glass beads and then the flask is agitated manually for about 20-30 min. The purpose of agitation is to induce trauma to the white blood cells and to make contact between the traumatized cells and the serum factor. After incubating the flask at 37C for 30 min, the sample is centrifuged for 1 min at 8000g or 700g for 5 min. The Buffy coat is separated from the centrifuged sample and it is stained with either Wright′s stain or Giemsa stain and observed under a microscope.
LE cells, if present in large numbers, are highly suggestive of SLE, but it is also seen in other chronic inflammatory autoimmune diseases like chronic discoid LE, systemic sclerosis and rheumatoid arthritis. A positive LE cell is a feature of drug-induced LE, like procainamide-induced lupus. [67]
Prozone phenomenon
The prozone phenomenon refers to a false-negative serologic test in which very high antibody titers interfere with the antigen-antibody lattice network necessary to visualize a positive flocculation test venereal disease research laboratory/treponema pallidum haemagglutination (VDRL/TPHA). [68] Thus, in the presence of this phenomenon, a higher dilution of the serum sample should be made to elicit a true-positive flocculation test.
The prozone phenomenon occurs in 1-2% of cases of secondary syphilis, leading to false-negative results in the serum sample. [69] Hence, when clinical evidence for secondary syphilis is strong and serum VDRL is reported negative, the VDRL test should be repeated in higher dilutions to rule out the prozone phenomenon.
In the current scenario, human immunodeficiency virus (HIV)-positive patients exhibit B-cell dysregulation, which leads to hyper-gammaglobulinemia, giving high antibody titers, thereby showing the prozone phenomenon. Hence, there is an increase in the prevalence of the prozone phenomenon in patients co-infected with syphilis than in syphilitic patients who are negative for HIV.
The prozone phenomenon is also seen in other infections like brucellosis, typhoid and hepatitis B. [70]
Reynold′s Braude phenomenon [Figure - 5]
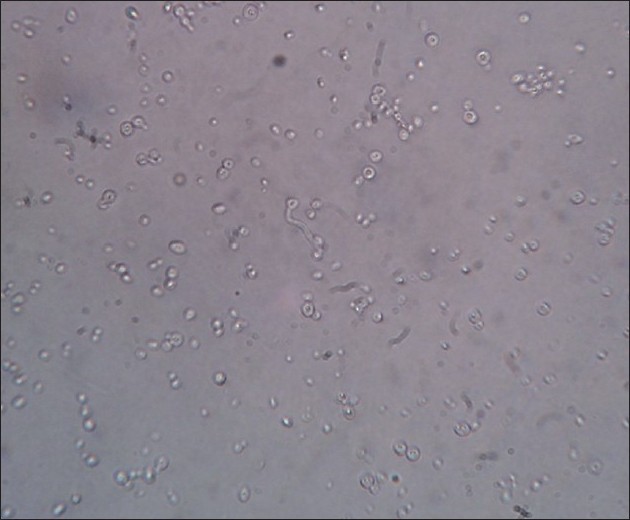 |
| Figure 5: Reynold's Braude phenomenon: germ tube formation is seen after incubating the ATCC strain of Candida albicans in human plasma for 2 h (×40) |
Incubation of the culture of Candida species with human or sheep serum at 37ºC produces germ tubes within 2-4 h. [71] This is called as Reynold′s Braude phenomenon. Germ tubes are seen to be originating from the pseudohyphae of Candida albicans. The significance of this phenomenon is that it helps to distinguish pathogenic C. albicans and C. dublinensis from other pathogenic strains like C. pseudotropicalis and C. parapsilosis. [72]
Epitope spreading phenomenon
Epitope spreading phenomenon explains the progressive formation of antibodies against more than one antigenic determinant during the course of an autoimmune disease. As the disease progresses, progressive tissue injury caused by primary antibodies results in the unmasking of neighbouring proteins to which secondary antibody responses are generated. In pemphigus vulgaris, epitope spreading occurs as a result of an immune response against the endogenous target antigens secondary to the release of self-antigen during the chronic autoimmune response. [73]
Antigen-specific autoimmune responses can spread to different epitopes on one protein (intramolecular epitope spreading) or to epitopes on other structural proteins (intermolecular epitope spreading). The formation of new antibodies may result in an atypical clinical picture. For example, the transformation of pemphigus foliaceous into pemphigus vulgaris or change from the mucosal form of pemphigus vulgaris to mucocutaneous form.
Besides pemphigus vulgaris, this mechanism is also postulated to play a role in the pathogenesis of pemphigus foliaceus, [73] paraneoplastic pemphigus [74] and lichen planus pemphigoides. [75]
Most of the phenomena deal with the pathogenetic processes involved with disease evolution. Understanding the mechanisms behind these phenomena improves our understanding of the concerned diseases and helps the physician in the rational management of the diseases.
Acknowledgment
The authors would like to thank Dr. Milind Ubale, Asssistant Professor, Department of Microbiology, Seth GS Medical College and KEM Hospital, for providing the images.
| 1. |
Merck source. Dorland′s Medical Dictionary for Healthcare Consumers. Available from: http://www.mercksource.com/pp/us/cns/cns_hl_dorlands_split.jsp?pg=/ppdocs/us/common/dorlands/dorland/nine/000955785.htm . [accessed on 2010 Jan 3].
[Google Scholar]
|
| 2. |
Miller RAW. The Koebner phenomenon. Int J Dermatol 1982;21:192-7.
[Google Scholar]
|
| 3. |
Pedace FJ, Muller SA, Winkelmann RK. The biology of psoriasis: An experimental study of the Koebner phenomenon. Acta Derm Venereol 1969;49:390-400.
[Google Scholar]
|
| 4. |
Kuner N, Hartschuh W, Khan-Durani B. Heinrich Kobner and the "isomorphic phenomenon". History and review of the literature. Hautarzt 2003;54:274-8.
[Google Scholar]
|
| 5. |
Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol 1990;29:401-10.
[Google Scholar]
|
| 6. |
Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol 2004;70:187-9.
[Google Scholar]
|
| 7. |
Wolf R, Brenner S, Ruocco V, Filioli FG. Isotopic response. Int J Dermatol 1995;34:341-8.
[Google Scholar]
|
| 8. |
Shemer A, Weiss G, Trau H. Wolf′s isotopic response: A case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol 2001;15:445-7.
[Google Scholar]
|
| 9. |
Fernandez-Redondo V, Amrouni B, Varela E, Toribio J. Granulomatous folliculitis at sites of herpes zoster scars: Wolf′s isotopic response. J Eur Acad Dermatol Venereol 2002;16:628-30.
[Google Scholar]
|
| 10. |
Sandhu K, Saraswat A, Handa S. Multiple epidermoid cysts occurring at site of healed herpes zoster in a renal transplant recipient: An isotopic response? Clin Exp Dermatol 2003;28:555-6.
[Google Scholar]
|
| 11. |
Mehregan AH. Licheniod and poikilodermatous tissue reactions. In: Pinkus′s guide to histopathology. 4th ed. Connecticut: Appleton-Century-Crofts; 1986. p. 129.
[Google Scholar]
|
| 12. |
Juneja M. Nikolskiy sign revisited. J Oral Sci 2008;50:213-4.
[Google Scholar]
|
| 13. |
International Study Group for Behcet′s disease. Criteria for the diagnosis of Behcet′s disease. Lancet 1990;335:1078-80.
[Google Scholar]
|
| 14. |
Findlay GH, Merwe LW. The Meirowsky phenomenon. Br J Dermatol 1966;78:572-6.
[Google Scholar]
|
| 15. |
James WD, Berger TG, Elston DM, editors. Dermatoses resulting from physical factors. In: Andrew′s Diseases of the skin Clinical Dermatology, 10th ed. Canada: WB Saunders; 2006. p. 27-9.
[Google Scholar]
|
| 16. |
Meyerson LB. A peculiar papulosquamous eruption involving pigmented nevi. Arch Dermatol 1971;34:510-2.
[Google Scholar]
|
| 17. |
Ramon R, Silvestre JF, Betloch I, Banuls J, Botella R, Navas J. Progression of Meyerson′s naevus to Sutton′s naevus. Dermatology 2000;200:337-8.
[Google Scholar]
|
| 18. |
Dawn G, Burden AD. Meyerson′s phenomenon around a seborrheic keratosis. Clin Exp Dermatol 2002;27:73.
[Google Scholar]
|
| 19. |
Crovato F, Nazzari G. Halo dermatitis around molluscum contagiosum: Meyerson′s phenomenon? Br J Dermatol 1994;131:452.
[Google Scholar]
|
| 20. |
Tegner E, BjoÈrnberg A, Jonsson N. Halo dermatitis around tumours. Acta Dermatol Venereol (Stockh) 1990;70:31-4.
[Google Scholar]
|
| 21. |
Krischer J, Pechere Salomon D, Harms M, Chavaz P, Saurat JH. Interferon alfa-2b-induced Meyerson′s nevi in a patient with dysplastic nevus syndrome. J Am Acad Dermatol 1999;1:105-6.
[Google Scholar]
|
| 22. |
Costa Carvalho IM, Kawano LB, Pereira CP, Carmo Nogueira LS. Lucio′s phenomenon: A case report and review of the literature. Int J Dermatol 2005;44:566-71.
[Google Scholar]
|
| 23. |
Harter P, Trinh TKMD. Formes escarrotiques d′erythema nodosum leprosum et leurs relations avec le phénomene de Lucio. Bull Soc Pathol Exot 1962;55:993-1024.
[Google Scholar]
|
| 24. |
Latapi F, Zamora AC. The "spotted" leprosy of Lucio (La lepra "manchada" de Lucio); An introduction to its clinical and histological study. Int J Lepr 1948;16:421-37.
[Google Scholar]
|
| 25. |
Saoji V, Salodkar A. Lucio leprosy with Lucio phenomenon. Indian J Lepr 2001;73:267-72.
[Google Scholar]
|
| 26. |
Rea TH, Ridley DS. Lucio′s phenomenon: A comparative histological study. Int J Lepr 1979;47:161-6.
[Google Scholar]
|
| 27. |
Buffon LP, Leal R, Vidigal MR, et al. Lucio′s phenomenon (erythema necroticans) in pregnancy: A case report and an overview. An Bras Dermatol 2001;76:441-8.
[Google Scholar]
|
| 28. |
Wikipedia. Ichthyosis bullosa of Siemens. Available from: http://en.wikipedia.org/wiki/Ichthyosis_bullosa_of_Siemens. [accessed on 2010 Mar 2].
[Google Scholar]
|
| 29. |
Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? A study of 142 cases. J Cutan Pathol 1995;22:342-8.
[Google Scholar]
|
| 30. |
Sutton RL, An unusual variety of vitiligo (Leukoderma Acquisitum Centrifugum). J Cutan Dis 1916;34:797.
[Google Scholar]
|
| 31. |
Rhodes AR, Grichnik JM, Sober AJ. Benign neoplasias and Hyperplasias of melanocytes. In: Wolff K, Goldsmith LA, Katz SI, Gilchrist BA, Paller AS, Leffell DJ, editors. Fitzpatrick′s Dermatology in General medicine, 7 th ed. New York: McGraw-Hill Book; 2008. p. 1099-107.
[Google Scholar]
|
| 32. |
Van der Steen P, Happle R. Castling phenomenon. Eur J Dermatol 1992;2:151-3.
[Google Scholar]
|
| 33. |
Happle R, Kalveran K, Buchner U, Echternacht-Happle K, Goggelman W, Summer KH. Contact allergy as a therapeutic tool for alopecia areata: Application of squaric acid dibutylester. Dermatologica 1980;161:289-97.
[Google Scholar]
|
| 34. |
Orecchia G. The "castling" phenomenon: A possible explanation. Eur J Dermatol 1994;4:161.
[Google Scholar]
|
| 35. |
Kasabach HH. Merriti KK. Capillary hemangioma with extensive purpura. Am J Dis Child 1940:59:1063-70.
[Google Scholar]
|
| 36. |
Warrel RP. Kempin SJ. Benua RS, Reiman RE, Young CW. Intratumoral consumption of indium-111 labeled platelets in a patient with hemangiomatosis and intravascular coagulation (Kasabach-Merritt syndrome). Cancer 1983;52:2256-60.
[Google Scholar]
|
| 37. |
Seo SK, Suh JC, Na GY, Kim IS, Sohn KR. Kasabach Merritt syndrome: Identification of platelet trapping in a tufted angioma by immunohistochemistry technique using monoclonal antibody to CD61. Pediatr Dermatol 1999;16:392-4.
[Google Scholar]
|
| 38. |
Koerper MA, Addiego JE, deLorimier AA, Lipow H, Price D, Lubin BH. Use of aspirin and dipyridamole in children with platelet trapping syndromes. J Pediatr 1983;102:311.
[Google Scholar]
|
| 39. |
Atherton DJ, Moss C. Naevi and other developmental defects. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook′s Textbook of Dermatology, 7 th ed. Oxford: Blackwell Publishing; 2004. p. 15.57-15.60
[Google Scholar]
|
| 40. |
Parischa JS, Khaitan BK. In: Clinical methods in Dermatology and Venereology, 5th ed. New Delhi: Mehta Publishers; 2005. p. 107.
[Google Scholar]
|
| 41. |
Kryst L, Wanyura H. Hoigne′s syndrome: Its course and symptomatology. J Max Surg 1979;7:320-6.
[Google Scholar]
|
| 42. |
Batchelor R, Horne G, Rogerson H. An Unusual Reaction to Procaine Penicillin in Aqueous Suspension. The Lancet 1951;195-8.
[Google Scholar]
|
| 43. |
Bjornberg A, Selstam J. Acute psychotic reaction after injection of procaine penicillin. Acta Psych Et Neurol Scand 1960;35:129-39.
[Google Scholar]
|
| 44. |
Wikipedia. Bell′s phenomenon. Available from: http://en.wikipedia.org/wiki/Bell′s_phenomenon#cite_ref-0. [accessed on 2010 Mar 9].
[Google Scholar]
|
| 45. |
Jones DH. Bell′s phenomenon should not be regarded as pathognomonic sign. BMJ 2001;323:935.
[Google Scholar]
|
| 46. |
Klippel JH. Raynaud′s phenomenon. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick′s Dermatology in General Medicine, 6 th ed. United States of America: McGraw Hill; 2003. p. 1763.
[Google Scholar]
|
| 47. |
Dowd PM. Reactions to cold. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook′s Textbook of Dermatology, 7 th ed. Oxford: Blackwell Publishing; 2004. p. 23.12.
[Google Scholar]
|
| 48. |
Kaya KI, Tiftik N, Tursen U, Ikizoglu U, Yalcin A. Ultraviolet recall phenomenon associated with methotrexate and cytarabine. J Eur Acad Dermatol Venereol 2000;20:353-4.
[Google Scholar]
|
| 49. |
Camidge R, Price A. Radiation recall dermatitis may represent the Koebner phenomenon. J Clin Oncol 2002;20:4130.
[Google Scholar]
|
| 50. |
Sehgal VN, editor. Psoriasis. In: Textbook of Clinical Dermatology 4 th ed. New Delhi: Jaypee Brothers Medical Publishing; 2006. p. 128.
[Google Scholar]
|
| 51. |
Maldonado RR, Covarrubias LO. Nutritional diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology, 2 nd ed. Spain: Mosby Elsevier; 2008. p. 667.
[Google Scholar]
|
| 52. |
James WD, Berger TG, Elston DM, editors. Cutaneous Vascular Diseases. In: Andrews′ Diseases of the Skin Clinical Dermatology, 10 th ed. Philadelphia: Saunders Elsevier; 2006. p. 824.
[Google Scholar]
|
| 53. |
Neligan GA, Strang LB. A "harlequin" colour change in the newborn. Lancet 1952;263:1005-7.
[Google Scholar]
|
| 54. |
Mortenson O. Harlequin color change of newborn. Acta Obstet Gynecol Scand 1959;38:352.
[Google Scholar]
|
| 55. |
Harris JE, Seykora JT, Lee RA. Renbok phenomenon and contact sensitization in a patient with alopecia universalis. Arch Dermatol 2010;146:422-5.
[Google Scholar]
|
| 56. |
Ito T, Hashizume H, Takigawa M. Contact immunotherapy- induced Renbok phenomenon in a patient with alopecia areata and psoriasis vulgaris. Eur J Dermatol 2010;20:126-7.
[Google Scholar]
|
| 57. |
Sulzberger-Chase phenomenon. Dorlands medical Dictionary. Available from: http://www.mercksource.com/pp/us/cns/cns_hl_dorlands_split.jsp?pg=/ppdocs/us/common/dorlands/dorland/six/000081582.htm. [accessed on 2010 Jul 6].
[Google Scholar]
|
| 58. |
Desensitization (Medicine). Wikipedia. Available from: http://en.wikipedia.org/wiki/Desensitization_(medicine). [accessed on 2010 Jul 6].
[Google Scholar]
|
| 59. |
Miller JJ, Roling D, Margolis D, Guzzo C. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol 1999;41:546-9.
[Google Scholar]
|
| 60. |
Kwangsukstith C, Vanittanakom N, Khanjanasthiti P, Uthammachai C. Cutaneous sporotrichosis in Thailand: First reported case. Mycoses 1990;33:513.
[Google Scholar]
|
| 61. |
Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol 2008;35:979-88.
[Google Scholar]
|
| 62. |
Larsson A, Hammarstrom L, Nethander G, Sjogren S. Multiple lesions of the lip exhibiting the ′Jadassohn phenomenon′: Review of literature and case report. Br J Dermatol 1977;96:307-12.
[Google Scholar]
|
| 63. |
Mitchelle RE. Squamous cell carcinoma developing in the Jadasshohn phenomenon: A case report Aust. J Dermatol 1975;16:79-82.
[Google Scholar]
|
| 64. |
Usui K, Ochiai T, Nishio H, Togo K, Yamagata M. Apocrine gland carcinoma of the mammary skin concomitant with pagetoid phenomenon. J Dermatol 2010;37:350-4.
[Google Scholar]
|
| 65. |
Hargraves MM, Richmond H, Morton R. Presentation of two bone marrow elements: The ′tart′ cell and the ′LE′ cell. Proc Staff Meetings Mayo Clin 1948;23:26-8.
[Google Scholar]
|
| 66. |
Holman HR. The LE cell phenomenon. Ann Rev Med 1960;11:231-42.
[Google Scholar]
|
| 67. |
Condemi JJ, Blomgren SE, Vaughan JH. Procainamide induced LE. Bull Rheum Dis 1970;20:604.
[Google Scholar]
|
| 68. |
Ferreira M, Teixeira M, Sanches M, Selores M. Infiltrated plaques on the face and back. Indian J Dermatol Venereol Leprol 2007;73:283-4.
[Google Scholar]
|
| 69. |
Berkowitz K, Baxi L, Fox HE. False negative syphilis screening: The prozone phenomenon, non-immune hydrops and diagnosis of syphilis during pregnancy. Am J Obstet Gynecol 1990;163:975-7.
[Google Scholar]
|
| 70. |
Jurado RL, Campbell J, Martin PD. Prozone phenomenon in secondary syphilis: Has its time arrived? Arch Intern Med 1993;153:2496-8.
[Google Scholar]
|
| 71. |
Chander J. Candidiasis. A textbook of medical mycology. New Delhi: Interprint; 1996. p. 131.
[Google Scholar]
|
| 72. |
Rimek D, Fehse B, Göpel P. Evaluation of Mueller-Hinton-agar as a simple medium for the germ tube production of Candida albicans and Candida dubliniensis. Mycoses 2008;51:205-8.
[Google Scholar]
|
| 73. |
Tchernev G, Orfanos CE. Antigen mimicry, epitope spreading and the pathogenesis of pemphigus. Tissue Antigens 2006;68:280-6.
[Google Scholar]
|
| 74. |
Chan L. Epitope spreading in paraneoplastic pemphigus: Autoimmune induction in antibody mediated blistering skin diseases. Arch Dermatol 2000;136:663-4.
[Google Scholar]
|
| 75. |
Inamdar AC, Palit A. Lichen planus and lichenoid disorders. In: Valia RG, Valia AR, editors. IADVL Textbook of Dermatology, 3 rd ed. Mumbai: Bhalani Publishing House; 2008. p. 1075-6.
[Google Scholar]
|
Fulltext Views
39,823
PDF downloads
9,561





