Translate this page into:
Photochemotherapy (PUVA) in psoriasis and vitiligo
Correspondence Address:
Smitha Prabhu
Department of Dermatology and Venereology, Kasturba Medical College, Manipal University, Manipal
India
| How to cite this article: Shenoi SD, Prabhu S. Photochemotherapy (PUVA) in psoriasis and vitiligo . Indian J Dermatol Venereol Leprol 2014;80:497-504 |
Abstract
Phototherapy with photochemotherapy (PUVA) is a well-known and well-studied modality for the treatment of psoriasis, which involves systemic or topical administration of chemicals known as psoralens and administration of ultraviolet light in increasing dosages after requisite time gap. PUVA is also used in the treatment of widespread vitiligo with moderately good results, though it is being surpassed by ultraviolet B (UVB), which is equally or slightly more efficacious with fewer side effects. PUVA induces repigmentation by varying mechanisms such as stimulation of melanogenesis, immunomodulation and activation of growth factors, though the exact mechanism is still speculative. There are various studies evaluating the efficacy of PUVA in psoriasis as well as in vitiligo, either alone or in combination with other immunosuppressants like azathioprine and calcipotriene.Introduction
Photochemotherapy (PUVA) is treatment involving the use of psoralen, an exogenous photosensitizer followed by ultraviolet A (UVA) irradiation. Both components are essential for clinical improvement as either of them used singly is not beneficial.
The history of PUVA dates back to 2000 BC when psoralen containing plants were used in Egypt. In 1974, Parish successfully introduced a treatment combining 8-methoxypsoralen (8MOP) and UVA called PUVA using the then newly developed Henselar high intensity artificial UVA light. [1] The classic multicentric studies by Melski et al.[2] in Europe and Henselar et al.[3] in the United States established the efficacy of PUVA and heralded a novel therapeutic approach in psoriasis.
Treatment of vitiligo, especially of wide-spread, recalcitrant disease is still prolonged and difficult and needs tenaciousness and patience on the part of the patient as well as the dermatologist. Phototherapy is the cornerstone of treatment of widespread vitiligo. PUVA has been used widely for treatment of widespread recalcitrant vitiligo in adults, though it is being increasingly surpassed by narrow band UVB (NB-UVB).
Rationale and scope
Psoriasis: PUVA has been found to be effective in psoriasis in well-conducted studies and randomized controlled trials. It is a relatively safe modality for the treatment of psoriasis, which is a chronic disease with remissions and relapses.
Vitiligo: PUVA and PUVASOL therapy are usually preferred for widespread vitiligo in adults which is not amenable to topical or other modalities of treatment. PUVA is known to induce repigmentation of lesions, but has to be given for a prolonged duration with at least 100-200 sessions given at least a day apart, 2-3 times a week. Repigmentation of varying degrees has been achieved, but sustained repigmentation is difficult to achieve.
Chemistry of psoralens
Psoralen and many of its derivatives are naturally occurring tricyclic furocoumarins. The derivative most widely used in PUVA is 8MOP (methoxsalen), which is principally of plant origin, but is available as a synthetic drug. 4, 5, 8-trimethyl psoralen (TMP, trioxsalen) is a synthetic compound, which is less phototoxic after oral administration and is primarily used for the treatment of vitiligo. 5-methoxypsoralen (5MOP, Bergapten), which has a lower potential for phototoxicity is also sometimes used.
Pharmacology
After oral intake of 8MOP, photosensitivity develops after 1 h, reaches a peak at about 2 h and disappears after about 8 h. [4]
The intestinal absorption rate of psoralens depends upon the physical characteristics of the preparation, concomitant food intake, and individual factors. Dissolved preparations (e.g, soft gelatin capsules) are better absorbed than micronized, crystalline formulations (e.g, hard gelatin capsules) and yield peak serum levels in a relatively reproducible time in all subjects. Food intake retards and decreases the absorption of psoralens. In the blood, 75-80% of methoxsalen is reversibly bound to serum albumin and is distributed to all organs. In the absence of UVA exposure, the binding is short lived and the drug is rapidly metabolized in the liver and excreted with urine as inactive metabolites. Drugs that induce cytochrome P-450 enzymes accelerate the metabolism of methoxsalen and may decrease the biologic effect of PUVA.
The inter-patient variability in the response to PUVA may be explained based on some biochemical markers, for example, glutathione S-transferase genotype is associated with sensitivity to psoralen-UVA photochemotherapy. [5] Individuals with a high clearance and low maximum serum concentration usually show reduced sensitivity to PUVA. [6]
Photochemistry of psoralens
Psoralens enter the cells and intercalate between DNA base pairs. On exposure to UVA, psoralens absorb photons, become chemically activated and covalently bind to DNA base pairs forming crosslinks. The DNA crosslinks have antiproliferative, antiangiogenic, apoptotic, and immunosuppressive effects. [7] The immunosuppressive effects include alteration in cytokines and antigen presenting cells with reduced expression of adhesion molecules and lymphocyte apoptosis. [8],[9],[10],[11]
The exact mechanism of pigment induction by PUVA in vitiligo is still speculative. Psoralens stimulate melanogenesis. The photoconjugation of psoralens in melanocyte DNA leads to mitosis, replication and proliferation of melanocytes, increased number of melanosomes and their further transfer to keratinocytes. Stimulation of cyclic adenosine monophosphate (cAMP) activity by PUVA leads to increased synthesis of tyrosine. PUVA also affects immunological processes and may induce a suppressor T cell population and release IL-10 which is important for differentiation and activation of T regulatory cells that may suppress the auto-immune stimulus responsible for melanocyte destruction. [12] PUVA also induces basic fibroblast growth factor (bFGF) and hepatocyte growth factor, which may aid in regrowth and migration of follicular melanocytes to the basal layer of skin. [13]
PRINCIPLES OF PUVA
The main principle of PUVA is producing a phototoxic reaction followed by pigmentation. PUVA-induced erythema usually appears 36-48h after exposure to UVA radiation and peaks at 48-96h or even up to 120h. [14] PUVA-induced pigmentation can occur even in the absence of erythema.
UVA Source
For PUVA, mainly broad band, high intensity UVA sources are used. The action spectrum is reported to be between 320 and 380 nm. Most UVA treatment units currently available are equipped with fluorescent bulbs. Because accurate dosing is necessary for safe therapy, the spectral power distribution of the UVA system has to be known and UVA dosing must be adjusted accordingly. UVA doses are given in J/cm 2 , usually measured with a photometer with a maximum sensitivity at 360 nm. [15]
Indications for PUVA
In psoriasis
- Psoriasis involving >20% body surface area
- Unresponsiveness to topical therapy/TL-O1 therapy
- Localized psoriasis of hands and feet (hand/foot unit)
- Localized disease not responding to other modalities of therapy.
In vitiligo
- Vitiligo involving more than 10% body surface area
- Patients with localized disease not responding to topical PUVA or other modalities of treatment.
Exclusion criteria
- Children aged less than 10 years. (Although in exceptional circumstances, younger age groups may be considered for treatment provided regular ophthalmologic evaluation is done to rule out ocular toxicity)
- Pregnancy and lactation
- People suffering from photosensitivity disorders.
Patient selection in vitiligo
This is important for obtaining optimal results. Darker skinned people respond better than those who are fair skinned. Although head and neck lesions and lesions on hairy parts of the body respond best, certain areas such as lips, dorsae of hands, acral parts, bony prominences, palms, soles, and nipples are refractory to treatment. Segmental vitiligo may or may not respond. In generalized vitiligo, though other areas may respond to treatment, the above-mentioned problem areas remain unresponsive. Duration of disease does not affect the response rate to PUVA. A 10-year retrospective study has concluded that there is ′need for careful patient counseling before PUVA therapy as this treatment seldom achieves extensive repigmentation that is cosmetically acceptable, and treatment response is often followed by relapse.′ [16] Patients with vitiligo affecting more than 30-40% body surface area (BSA) do not respond well to medical therapy like PUVA/PUVASOL and should be counseled prior to inclusion into therapy, explaining to them the possibility of inadequate response to treatment.
TREATMENT METHODOLOGY [17]
There are two protocols that are followed in psoriasis:
- US protocol: The first treatment exposure dose is based on the skin type and patients are treated either twice or thrice a week. Dose increments range from 0.5 to 1.5 J/cm 2 depending on erythema production and therapeutic response
- European protocol: The first treatment is administered after determination of the individual′s minimum phototoxic dose (MPD) and the initial UVA dose is the patient′s MPD. The MPD is defined as the minimal dose of UVA delivered to the skin after ingestion of 8MOP that produces a barely perceptible, well-defined erythema when small template test areas are exposed to increasing doses of UVA ranging from 0.5 to 5 J/cm 2 . The erythema readings are performed 72 h after testing when the phototoxicity has reached a peak. Two treatments per week are given using 0.6 mg/kg 8MOP or 1.2 mg/kg 5MOP with 0.5 J/cm 2 increment at each visit till a mild erythema occurs on vitliginous areas. [18] Lesions of the lower limbs tend to respond slower so extra treatment may be needed. These areas are irradiated with an additional 0.5-5 J/cm 2 , the dose being gradually increased.
The IADVL therapeutic guidelines committee recommends a modified US regime suited to Indian patients, in which the initial treatment dose is 2-3 J/cm 2 and is subsequently increased by 0.5 J/cm 2 provided the patient has not developed erythema or burning sensation over the apparently normal skin. This is so for both psoriasis and vitiligo.
The general principle is to hold the dose of the drug and interval between drug intake constant and to vary the UVA dose according to the sensitivity of the patient. 8MOP in a dose of 0.6-0.8 mg/kg body weight is administered orally followed by whole body irradiation after 1-3 h. The initial dose of UVA is predetermined by either skin typing or phototoxicity testing. Repeated exposures are necessary to clear PUVA responsive diseases, and as pigmentation develops, UVA doses have to be increased.
Although ideally 8MOP should be taken on an empty stomach, in practice, it is taken after food mainly to prevent nausea.
Psoriasis
Initial treatment (clearance phase): Treatments are generally given 2-3 times a week with at least 48h intervals between treatments. If mild erythema develops, the dose may either be reduced or kept constant but if moderate-to-severe erythema develops, treatment is deferred.
The average number of exposures required for clearing is about 20, but varies from 15 to 25. The final clearance dose of UVA radiation is about 5-20 J/cm 2 depending upon the skin type.
In psoriasis, the mean total cumulative UVA dose needed for all skin types to clear was 103 and 79 J/cm 2 , respectively, in two European trials. [3] The mean cumulative UVA dose was considerably higher (245 J/cm 2 ) for the US trial. [2] Psoriasis of nails, palms, and soles does not respond well to oral PUVA.
Maintenance treatment: The last dose of clearance phase is kept constant and the frequency of treatments is slowly reduced to as low as once a month. [2]
If maintenance PUVA treatment is given for 2-3 months and then stopped in patients who are still clear, it has been shown that majority of patients remain free of disease for at least 6-12 months. [15]
Treatment of relapses: If significant relapse of the disease occurs after treatment discontinuation or during the maintenance phase, it is appropriate to resume a clearance schedule. For minor recurrences occurring during the maintenance phase, the frequency of treatments may be increased until disease control is achieved.
There are studies showing that twice weekly PUVA is as effective as the conventional thrice weekly regimen. [19],[20] This may help in improving patient compliance as well as reducing the total cumulative UVA dose.
PUVASOL
PUVASOL stands for psoralen and UVA obtained by solar light as sunlight is a rich source of UVA, PUVASOL is advised for those patients who cannot visit the hospital for phototherapy. A major disadvantage of solar irradiation as a light source is the difficulty in quantifying UV light. The total amount of UVA reaching the skin at any one time varies widely depending on the season, time of the day, latitude, and conditions of the atmosphere. Other disadvantages are lack of privacy, difficulty in monitoring the dose of ultraviolet rays and in addition to UVA, ultraviolet B (UVB), infrared rays and visible light, which are not needed for PUVA therapy may lead to undesirable effects. UVB in sunlight can increase the thickness of epidermis and makes the sun-exposed skin leathery and may interfere with the effectiveness of light therapy. [21] According to a study conducted by Balasaraswathy et al., the best time of the day for PUVASOL is between 9.15-11.15 a.m. and 2.30-3.30 p.m. There is minimal unwanted exposure to UVB and infrared light at these times. [22]
Among the sunglasses available in India, B2 TORID and Green 80 glasses give the best protection from UVA. [23]
Creating a solarium to utilize the UVA in sunlight and cutting off the UVB by having glass roof is another alternative to artificial units. [24]
In two Indian studies, nearly 55% and 63% of patients have shown good to excellent improvement with PUVASOL in psoriasis. [25],[26]
8MOP in the dose of 0.6 mg/kg body weight is administered after breakfast. Nearly 1.5-2h later, sun exposure is advised for 10 min. Treatment is carried out 2-3 times/week and time of exposure is increased by 5 min every week till a maximum of 30-45 min. Use of eye protective glasses and avoidance of further sun exposure for the next 8h is to be followed to prevent eye toxicity and darkening of the normal skin.
Treatment protocols in vitiligo [27],[28]
Vitiligo needs a larger number of treatments for clearance, as compared with psoriasis, if therapy is continued till repigmentation is achieved, which may last for months to years.
Oral 8MOP or TMP are commonly used. Dosage and frequency of administration is similar to that in psoriasis, the major difference being the need for prolonged treatment lasting for 150-200 sessions in vitiligo, and the difficulty in achieving complete or near-to-complete response.
PUVASOL: Here, oral TMP is preferable to 8MOP due to its weaker phototoxic effects. Treatment should be given twice to thrice weekly, with a minimum gap of 1 day between treatments. The duration of sun exposure is to be increased weekly until there is mild erythema of the involved area after which, that time can be kept constant. If there is no improvement even after 30-40 sittings, it should be discontinued.
Topical PUVA with 8MOP may be used in patients with smaller lesions involving less than 5% of body surface area. 0.01-0.1% 8MOP in a cream or lotion base is applied to the affected area and irradiation is done after 30 min. A weekly increment of 0.25 J/cm 2 or by 20% of the previous dose is given till onset of erythema. Application of sunscreen to the surrounding uninvolved skin can prevent undue tanning. TMP and 5MOP are more phototoxic topically. Though topical PUVASOL is generally avoided due to the greater phototoxic risk and frequent occurrence of painful blisters, it may be tried during rainy season. A total of 0.1% 8MOP is applied carefully over the vitiliginous patches (one part of commercially available 8MOP lotion can be diluted with nine parts of eau-de-cologne and used). Propylene glycol can also be used as a diluent. After 30 min, the patches are exposed to sunlight starting with 0.5-1 min. Treatment is done 2-3 times/week and duration of sun exposure should be slowly increased by 0.5-1 min every week till slight erythema appears after which the time is kept constant.
Studies on efficacy of PUVA in vitiligo are shown in [Table - 1]. For optimal and maximum pigment induction, prolonged therapy lasting for months is required with as many as 100-200 exposures given 2-3 times a week. Response is denoted by the occurrence of perifollicular repigmentation and 70% cases respond within 12-24 weeks. If there is no response even after approximately 50 sessions, PUVA should be discontinued.
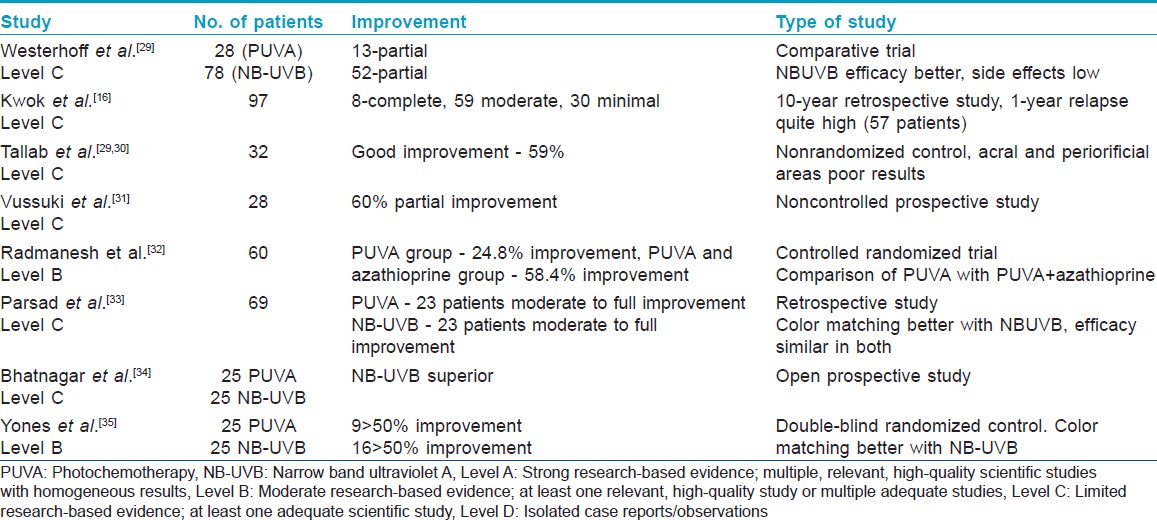
If response occurs and patient discontinues treatment, the newly acquired repigmentation may be lost. Completely repigmented areas may remain stable for more than 10 years. [36]
Combination with other modalities
- Calcipotriol and PUVA: Many studies show quicker response to treatment with more intense repigmentation, though acral vitiligo does not respond well. Concurrent topical calcipotriol may shorten the duration of UVA exposure thus leading to reduction of PUVA-induced side effects [3],[37],[38],[39],[40],[41] (Level B). However, there are isolated studies which do not demonstrate any beneficial effect of combined treatment over PUVA [42] (Level C)
- Concurrent low dose azathioprine also has been used to improve the efficacy of PUVA in vitiligo. [32]
Precautions during PUVA therapy [43]
- UV-blocking goggles are used to protect the eyes. If treatment is not required for facial involvement, the face is protected either by use of a broad spectrum sunscreen with an SPF of 50+ or a cloth barrier. Male genitalia are protected with the use of underwear or an athletic supporter
- Patients must protect their eyes after ingesting psoralen. Wraparound UV-blocking glasses, which give complete UVA photoprotection like B2 Torid and Green 80, should be worn when the patient is exposed to sunlight, from the time methoxsalen is ingested until sunset that same day
- If PUVA is to be given to the genitalia, exposure at 25% of the ideal dose can be safely undertaken
- Sun avoidance is advised to minimize pigmentation from natural sunlight. Excessive pigmentation may ultimately limit the effectiveness of PUVA therapy and require higher doses of UVA. The skin should be protected from natural sunlight through appropriate clothing and avoidance
- The amount of UVA emitted by common fluorescent lights is insufficient to activate psoralens. Thus, photoprotection is not required in home or office settings.
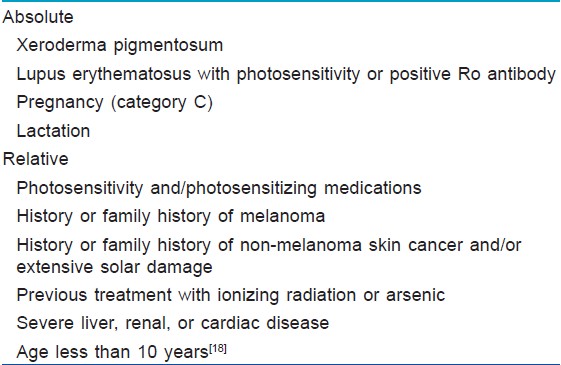
Contraindications to PUVA [44],[45] [Table - 2].
Side-Effects of PUVA
Short-term side effects
- Nausea and vomiting can be managed by taking the drug with food preferably of high fat content or milk. If it persists, dose can be reduced and/or antiemetics administered
- Erythema, pruritus and xerosis can be managed by emollients, antihistamines and other antipruritic agents
- Skin pain occurs due to phototoxic damage of dermal nerve endings and can be managed by gabapentin [46]
- Pigmentation: excessive darkening can occur with repeated treatments especially in dark skinned patients
- Others: Central nervous system (CNS) side effects such as headache, dizziness, depression, insomnia and hyperactivity may be seen. [47]
- Reactivation of herpes simplex
- Bronchoconstriction, drug fever, tachycardia
- Photo-onycholysis, [47],[48] melanonychia, [49] friction blisters and ankle edema. [50]
Long-term side effects
Photoaging: This occurs in all patients with Fitzpatrick skin types I to IV after long-term PUVA therapy. These changes are partially reversible upon early discontinuation of therapy. Skin types I and II have more marked changes than types III and IV.
The photoaging changes are similar to those produced by natural sunlight and include hyper- or hypopigmentation, telangiectasia, wrinkles, lentigines, and actinic keratosis. Hypertrichosis has been reported to occur in both men and women treated with long-term PUVA. [51]
Eye toxicity: This occurs as psoralens accumulate in the lens with an increased potential for cataract. But a 25-year prospective study of patients treated with PUVA from the large US cohort study did not demonstrate an increased risk of either visual impairment or cataract formation with increasing exposure to PUVA. [52]
Cutaneous malignancies: High cumulative exposure to oral PUVA is associated with a dose-related increase in the risk of nonmelanoma skin cancer, particularly squamous cell carcinoma (SCC). [53],[54],[55]
An increased risk of skin cancer with oral PUVA has not been demonstrated in the non-Caucasian population. [56]
Men exposed to PUVA have an increased risk of genital skin cancer. In a cohort of 892 men treated with PUVA, the incidence of invasive scrotal or penile squamous cell carcinoma was 53-fold higher than that expected in the general white population. [57]
The risk of melanoma following PUVA is controversial. [58],[59],[60]
Additional problems in vitiligo: The treatment may also stimulate pigmentation of normal skin which can accentuate the difference between normal and vitiliginous areas. There is a case report of occurrence of squamous cell carcinoma in vitiligo patients after long-term PUVA [61]
PUVA in Indian skin has a very good safety profile and, so far, cases of carcinogenesis or other serious side effects have not been reported, though by no means should we remain complacent, and should actively follow up the patients on long-term PUVA.
Monitoring patients during PUVA therapy:
- Liver function test may be done in patients prior to starting PUVA, if long-term treatment is planned. If baseline liver function tests (LFT) are normal, further routine monitoring is not required
- A pretreatment eye evaluation may be done, and repeated every 6 months. Any eye complaint in a patient while on PUVA should be promptly attended to
- Patients who are on PUVA for long may be periodically monitored for suspicious growths on the skin, though cutaneous malignancy has not been reported in Indian patients on PUVA.
Drug interactions: Phototoxic drugs (e.g, thiazides, tetracyclines, fluoroquinolones, phenothiazines, or sulfonamides) and topical preparations (e.g, anthralin or coal-tar) may augment the action of PUVA and increase the risk of acute phototoxic erythema so these may have to be discontinued.
Modifications of PUVA: These include clothes-on PUVA, [62] PUVA soaks, [63] bath PUVA, [64] turban PUVA, PUVA combs, which though mainly used in alopecia areata may be used for psoraisis and vitiligo of the scalp too, and blue point PUVA for nails.
Recommendations for PUVA in psoriasis and vitiligo
PUVA is a well-studied therapeutic entity in psoriasis with level of evidence being level A, whereas in vitiligo, though case control studies are abundant, there is no concrete multicentric randomized control trial suggesting the efficacy of PUVA in complete resolution of the lesions. Most studies and reports suggest a level of evidence B and C.
Special issues in the Indian situation
PUVA is well tolerated in Indians, the risk for malignancies being minimal to nil. In areas and setups where office or hospital administered PUVA is difficult logistically and financially, PUVASOL can be carried out with moderate efficiency.
Conclusion
PUVA is a well-known modality for the treatment of widespread psoriasis and vitiligo alike with a well-established efficacy in psoriasis as well as vitiligo, as suggested by many studies.
| 1. |
Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. New Engl J Med 1974;291:1207-11.
[Google Scholar]
|
| 2. |
Melski, JW, Tanenbaum L, Parrish JA, Fitzpatrick TB, Bleich HL. Oral methoxsalen photochemo therapy for the treatment of psoriasis: A cooperative clinical trial. J Invest Dermatol 1977;68:328-35.
[Google Scholar]
|
| 3. |
Henseler T, Hönigsmann H, Wolff K, Chrisophers E. Oral 8-methoxypsoralen photochemotherapy of psoriasis. The European PUVA study: A cooperative study among 18 European centres. Lancet 1981;317:853-7.
[Google Scholar]
|
| 4. |
Reynolds EF. In: Martinadale The extra Pharmacopia. 29 th ed. London: The Pharmaceutical Press; 1989. p. 920.
[Google Scholar]
|
| 5. |
Ibbotson SH, Dawer S, Dinkova-Kostova AT, Weidlich S, Farr PM, Ferguson J, et al. Glutathione S-transferase genotype is associated with sensitivity to psoralen-ultraviolet Aphotochemotherapy. Eur J Dermatol 2011;21:53-7.
[Google Scholar]
|
| 6. |
De Wolff F A, Thomas T V. Clinical pharmacokinetics of methoxsalen and other psoralens. Clin Pharmacokinet 1986;11:62-75.
[Google Scholar]
|
| 7. |
Ceoviæ R, Pasiæ A, Lipozenciæ J, Jakiæ-Razumoviæ J, Szirovicza L, Kostoviæ K. Antiproliferative, antiangiogenic and apoptotic effect of photochemotherapy (PUVA) in psoriasis patients. Coll Antropol 2007;31:551-6.
[Google Scholar]
|
| 8. |
Sethi G, Sodhi A. Role of p38 mitogen-activated protein kinase and caspases in UV-B-induced apoptosis of murine peritoneal macrophages. Photochem Photobiol 2004;79:48-54.
[Google Scholar]
|
| 9. |
Laing TJ, Richardson BC, Toth MB, Smith EM, Marks RM. Ultraviolet light and 8-methoxypsoralen inhibit expression of endothelial adhesion molecules. J Rheumatol 1995;22:2126-31.
[Google Scholar]
|
| 10. |
Singh TP, Schön MP, Wallbrecht K, Michaelis K, Rinner B, Mayer G, et al. 8-methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder. J Immunol 2010;184:7257-67.
[Google Scholar]
|
| 11. |
Johnson R, Staiano-Coico L, Austin L, Cardinale I, Nabeya Tsukifuji R, Krueger JG. PUVA treatment selectively induces a cell cycle block and subsequent apoptosis in human T-lymphocytes. Photochem Photobiol 1996;63:566-71.
[Google Scholar]
|
| 12. |
Falabella R, Barona MI. Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res 2008;22:42-65.
[Google Scholar]
|
| 13. |
Wu CS, Lan CC, Wang LF, Chen GS, Wu CS, Yu HS. Effects of psoralen plus ultraviolet A irradiation on cultured epidermal cells in vitro and patients with vitiligo in vivo. Br J Dermatol 2007;156:122-9.
[Google Scholar]
|
| 14. |
Ibbotson SH, Farr PM. The time-course of psoralen ultraviolet A (PUVA) erythema. J Invest Dermatol 1999;113:346-50.
[Google Scholar]
|
| 15. |
Srinivas CR, Shenoi SD, Pai S. Psoralens. Indian J DermatolVenereol Leprol 1997;63:276-87.
[Google Scholar]
|
| 16. |
Kwok YK, Anstey AV, Hawk JL. Psoralen photochemotherapy (PUVA) is only moderately effective in widespread Vitiligo: A 10-year retrospective study. Clin Exp Dermatol 2002;27:104-10.
[Google Scholar]
|
| 17. |
Hönigsmann H, Schwarz T. Photochemotherapy with psoralens. Chapter 134. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Bolognia: Dermatology. 2 nd ed. United States: Mosby Elsevier; 2008.
[Google Scholar]
|
| 18. |
British Photodermatology Group. British photodermatology group guidelines for PUVA. Br J Dermatol 1994;130:246-55.
[Google Scholar]
|
| 19. |
Buckley DA, Healy E, Rogers S. A comparison of twice-weekly MPD-PUVA and three times-weekly skin typing-PUVA regimens for the treatment of psoriasis. Br J Dermatol 1995;133:417-22.
[Google Scholar]
|
| 20. |
Sakuntabhai A, Sharpe GR, Farr PM. Response of psoriasis to twice weekly PUVA. Br J Dermatol 1993;128:166-71.
[Google Scholar]
|
| 21. |
Rai R, Srinivas CR. Phototherapy: An Indian perceptive. Indian J Dermatol 2007;52:169-75.
[Google Scholar]
|
| 22. |
Balasaraswathy P, Kumar U, Srinivas CR, Nair S. UVA and UVB in sunlight. Opitmal utilization of UV rays in sunlight for phototherapy. Indian J Dermatol 2002;68:198-201.
[Google Scholar]
|
| 23. |
Pai S, Srinivas RC, Gibbs N, Ferguson J. UVA transmission properties of sunglasses used by patients undergoing photochemotherapy. Indian J Dermatol Venereol Leprol 1991;57:270-1.
[Google Scholar]
|
| 24. |
Tiwari A, Srinivas CR, Musthafa M, Rai R, Surendran P. Installation of solarium-2000 for phototherapy. Indian J Dermatol Venereol Leprol 2003;69:10-1.
[Google Scholar]
|
| 25. |
Marquis L, Rangwala GM. Photochemotherapy of psoriasis with oral 8-methoxypsoralen (8-MOP) and solar irradiation (PUVASOL therapy). Indian J Dermatol Venereol Leprol 1980;46:297-8.
[Google Scholar]
|
| 26. |
Talwalkar PG, Gadgil RB, Obemi C, Parekh VD. Evaluation of 8-methoxypsoralen and solar ultraviolet light (PUVASOL) in psoriasis. Indian J Dermatol Venereol Leprol 1981;47:17-20.
[Google Scholar]
|
| 27. |
Anstey AV. Disorders of Skin Pigmentation. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of dermatology. 8 th ed. West Sussex: Wiley Blackwell Publishers; 2010. p. 58.50-2.
th ed. West Sussex: Wiley Blackwell Publishers; 2010. p. 58.50-2.'>[Google Scholar]
|
| 28. |
Phototherapy and photodynamic therapy. Chapter 239. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7 th edition. USA: The McGraw Hill Companies; 2008. p. 2256.
th edition. USA: The McGraw Hill Companies; 2008. p. 2256.'>[Google Scholar]
|
| 29. |
Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol 1997;133:1525-8.
[Google Scholar]
|
| 30. |
Tallab T, Joharji H, Bahamdan K, Karkashan E, Mourad M, Ibrahim K. Response of Vitiligo to PUVA therapy in Saudi patients. Int J Dermatol 2005;44:556-8
[Google Scholar]
|
| 31. |
Vussuki E, Ziv M, Rosenman D, David M. Longterm effects of PUVA therapy on Israeli patients with vitiligo. Harefuah 2006;145:483-5.
[Google Scholar]
|
| 32. |
Radmanesh M, Saedi K. The efficacy of combined PUVA and low-dose azathioprine for early and enhanced repigmentation in vitiligo patients. J Dermatolog Treat 2006;17:151-3.
[Google Scholar]
|
| 33. |
Parsad D, Kanwar AJ, Kumar B. Psoralen-ultraviolet A vs. narrow-band ultraviolet B phototherapy for the treatment of vitiligo. J Eur Acad Dermatol Venereol 2006;20:175-7.
[Google Scholar]
|
| 34. |
Bhatnagar A, Kanwar AJ, Parsad D, De D. Psoralen and ultraviolet A and narrow-band ultraviolet B in inducing stability in vitiligo, assessed by vitiligo disease activity score: An open prospective comparative study. J Eur Acad Dermatol Venereol 2007;21:1381-5.
[Google Scholar]
|
| 35. |
Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized double-blind trial of treatment of vitiligo: Efficacy of psoralen-UV-A therapy vs Narrowband-UV-B therapy. Arch Dermatol 2007;143:578-84.
[Google Scholar]
|
| 36. |
Hönigsmann H, Schwarz T. Bolognia: Dermatology. 2 nd ed. Chapter 134. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Photochemotherapy with psoralens. UK: Mosby Elsevier; 2008.
[Google Scholar]
|
| 37. |
Cherif F, Azaiz MI, Hamida AB, Dhari AB. Calcipotriol and PUVA as treatment for Vitiligo. Dermatol Online J 2003;9:4.
[Google Scholar]
|
| 38. |
Parsad D, Saini R, Verma N. Combination of PUVAsol and topical calcipotriol in vitiligo. Dermatology 1998;197;167-70.
[Google Scholar]
|
| 39. |
Yalçin B, Sahin S, Bukulmez G, Karaduman A, Atakan N, Akan T, et al. Experience with calcipotriol as adjunctive treatment for vitiligo in patients who do not respond to PUVA alone: A preliminary study. J Am Acad Dermatol 2001;44:634-7.
[Google Scholar]
|
| 40. |
Ermis O, Alpsoy E, Cetin L, Yilmaz E. The efficacy of psoralen plus ultraviolet A therapy for vitiligo enhanced by concurrent topical calcipotriol? A placebo-controlled double-blind study. Br J Dermatol 2001;145:472-5.
[Google Scholar]
|
| 41. |
Ameen M, Exarchou V, Chu AC. Topical calcipotriol as monotherapy and in combination with psoralen plus ultraviolet A in the treatment of vitiligo. Br J Dermatol 2001;145:476-9.
[Google Scholar]
|
| 42. |
Baysal V, Yildirim M, Erel A, Kesici D. Is the combination of calcipotriol and PUVA effective in Vitiligo? J Eur Acad Dermatol Venereol 2003;17:299-302.
[Google Scholar]
|
| 43. |
Richard EG, Morison W. Psoralen plus ultraviolet A (PUVA) photochemotherapy. Available from: http://www.uptodate.com/contents/psoralen-plus-ultraviolet-a-puva-photochemotherapy [last accessed on 2012 Jun 18, cited on 19.08.12].
[Google Scholar]
|
| 44. |
Morison WL. Phototherapy and Photochemotherapy of Skin Disease. 3 rd ed. New York: Taylor and Francis 2005.
[Google Scholar]
|
| 45. |
Morison WL. PUVA Photochemotherapy. In: Comprehensive dermatologic drug therapy. 2 nd ed, In: Wolverton SE, editor. Philadelphia: Elsevier; 2007.
[Google Scholar]
|
| 46. |
Zamiri M, Bilsland D. Treatment of bath PUVA-induced skin pain with gabapentin. Br J Dermatol 2004;151:516-7.
[Google Scholar]
|
| 47. |
Morison WL, Marwaha S, Beck L. PUVA-induced phototoxicity: Incidence and causes. J Am Acad Dermatol 1997;36:183-5.
[Google Scholar]
|
| 48. |
Mackie RM. Onycholysis occurring during PUVA therapy. Clin Exp Dermatol 1979;4:111-3.
[Google Scholar]
|
| 49. |
Ledbetter LS, Hsu S. Melanonychia associated with PUVA therapy. J Am Acad Dermatol 2003;48:S31-2.
[Google Scholar]
|
| 50. |
Prens EP, Smeenk G. Effect of photochemotherapy on the cardiovascular system. Dermatologica 1983;167:208-11.
[Google Scholar]
|
| 51. |
Rampen FH. Hypertrichosis in PUVA-treated patients. Br J Dermatol 1983;109:657-60.
[Google Scholar]
|
| 52. |
Malanos D, Stern RS. Psoralen plus ultraviolet A does not increase the risk of cataracts: A 25-year prospective study. J Am Acad Dermatol 2007;57:231-7.
[Google Scholar]
|
| 53. |
Stern RS, Thibodeau LA, Kleinerman RA, Parrish JA, Fitzpatrick TB. Risk of cutaneous carcinoma in patients treated with oral methoxsalen photochemotherapy for psoriasis. N Engl J Med 1979;300:809-13.
[Google Scholar]
|
| 54. |
Stern RS, Laird N, Melski J, Parrish JA, Fitzpatrick TB, Bliech HL. Cutaneous squamous-cell carcinoma in patients treated with PUVA. N Engl J Med 1984;310:1156-61.
[Google Scholar]
|
| 55. |
Nijsten TE, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen+ultraviolet A: A cohort study. J Invest Dermatol 2003;121:252-8.
[Google Scholar]
|
| 56. |
Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing non-melanoma skin cancer following long-term PUVA therapy. Int J Dermatol 2005;44:1016-21.
[Google Scholar]
|
| 57. |
Stern RS, Bagheri S, Nichols K. PUVA Follow Up Study. The persistent risk of genital tumors among men treated with psoralen plus ultraviolet A (PUVA) for psoriasis. J Am Acad Dermatol 2002;47:33-9.
[Google Scholar]
|
| 58. |
Morison WL, Baughman RD, Day RM, Forbes PD, Hoenigsmann H, Krueger GG, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol 1998;134:595-8.
[Google Scholar]
|
| 59. |
Lindelof B. Risk of melanoma with psoralen/ultraviolet a therapy for psoriasis. Do the known risks now outweigh the benefits? Drug Saf 1999;20:289-97.
[Google Scholar]
|
| 60. |
Stern RS, Nichols KT, Vakeva LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet a radiation (PUVA). The PUVA follow-up study. N Engl J Med 1997;336:1041-5.
[Google Scholar]
|
| 61. |
Park HS, Lee YS, Chun DK. Squamous cell carcinoma in vitiligo lesion after long-term PUVA therapy. J Eur Acad Dermatol Venereol 2003;17:578-80.
[Google Scholar]
|
| 62. |
Varma S, Ballambat SP, Balachandran C, Shenoi SD, Prabhu S. Clothes-on PUVA in psoriasis: Single blind randomized comparative trial on 21 patients. Indian J Dermatol Venereol Leprol 2004;70:152-5.
[Google Scholar]
|
| 63. |
O'Kane D, McLoone NM, Jenkinson H, Alderdice D, Badri M. Efficacy of topical PUVA soaks for palmoplantardermatoses: An audit. Photodermatol Photoimmunol Photomed 2008;24:279-84.
[Google Scholar]
|
| 64. |
Halpern SM, Anstey AV, Dawe RS, Diffely BL, Farr PM, Ferguson J. Guidelines for topical PUVA: A report of a workshop of the British Photodermatology Group. Br J Dermatol 2000;142:22-31.
[Google Scholar]
|
Fulltext Views
48,513
PDF downloads
5,525





