Translate this page into:
Phototherapeutic modalities pose no significantly increased risk of oxidative damage to DNA in dark skinned individuals
2 Department of Medical Biochemistry, Faculty of Medicine, Cairo University, Cairo, Egypt
Correspondence Address:
Heba Mohamed Mashaly
6 Gamal Salem Street, Off Mossadak Street, Dokki, Cairo
Egypt
| How to cite this article: Youssef R, Ossama S, Mashaly HM, Fathy M, Safwat M, Shaker O, El-Mofty M. Phototherapeutic modalities pose no significantly increased risk of oxidative damage to DNA in dark skinned individuals. Indian J Dermatol Venereol Leprol 2016;82:666-672 |
Abstract
Background: 8-oxoguanine, a major product of DNA oxidation, is considered a key parameter in measuring the carcinogenic effects of ultraviolet radiation. Objective: To assess and compare the carcinogenic potential of different photo (chemo) therapeutic modalities in photoresponsive skin diseases by measuring the levels of 8-oxoguanine in dark-skinned individuals before and after photo (chemo) therapy. Methods: A prospective, randomized controlled pilot study was conducted in 63 patients of skin types III–V with photo-responsive dermatoses including vitiligo, psoriasis and mycosis fungoides. Patients were divided into three groups; Group 1 (received narrowband ultraviolet-B), Group 2 (received psoralen plus ultraviolet-A) and Group 3 (received broadband ultraviolet-A). Biopsies were taken before and after phototherapy to measure 8-oxoguanine levels using enzyme-linked immunosorbent assay. Biopsies were also taken from the sun-protected skin in 21 controls subjects who had no dermatological disease. Results: Regardless of the disease, a significantly higher level of 8-oxoguanine was found after treatment when compared to the pre-treatment baseline levels; however, these levels were comparable to those in control subjects. A weakly significant positive correlation was found between cumulative dose and 8-oxoguanine levels following psoralen plus ultraviolet-A therapy. In controls, comparing the 8-oxoguanine levels between skin types III and IV showed significantly lower 8-oxoguanine in skin type IV. Conclusion: Therapeutic doses of ultraviolet radiation are relatively safe in dark skinned patients; however, minimizing the cumulative dose of phototherapeutic modalities (particularly psoralen plus ultraviolet-A) is recommended.Introduction
The popularity of phototherapy is on the rise, being an effective and relatively safe treatment option for many skin diseases. However, significant concerns exist regarding its carcinogenic potential.[1] Among the different phototherapeutic modalities, psoralen plus ultraviolet-A (PUVA) is the modality which has been maximally linked with the development of melanoma and non-melanoma skin cancer in white patients.[2]
The degree of carcinogenic potential of ultraviolet rays can be predicted fairly accurately by both patient-specific and phototherapeutic parameters. Pre-existing actinic damage, age, personal habits, previous or concomitant therapy and lifestyle are all patient-specific factors that influence the ultraviolet-induced carcinogenic risk; however, skin phototype is the major determinant with phototypes I–III having the most substantial risk.[3],[4] The type of phototherapy, dose delivered, duration of ultraviolet exposure, and number and frequency of sessions are also critical predictors of the carcinogenic risk.[5]
DNA photoproducts play a major role in induction of genotoxic effects by ultraviolet radiation. The DNA photolesion, 8-oxo-dihydro-guanine (oxidized base lesion) is produced by the action of reactive oxygen species that are formed in response to ultraviolet-A and ultraviolet-B. If this damaged DNA is not repaired before cell division, the gene mutation may be passed on to daughter cells.[6]
Despite several studies discussing ultraviolet-induced cancer risk in general, it is important to appropriately evaluate the carcinogenic potential of different types of phototherapy among the darker skinned population so as to accurately gauge the risk in this group.
Aim of study
The aim of this pilot study was to assess and compare the carcinogenic potential of different photo (chemo) therapeutic modalities, namely narrowband ultraviolet-B, low dose broadband ultraviolet-A and psoralen plus ultraviolet-A in darker skinned individuals (skin phototype III–V) with photoresponsive dermatoses by measuring 8-oxoguanine levels before and after photo (chemo) therapy.
Methods
Study design
This was a prospective randomized controlled pilot study.
Patients
Sixty-three patients of both sexes and varying ages with clinically and histopathologically proven vitiligo, psoriasis and mycosis fungoides were randomly selected from the dermatology outpatient clinic of Kasr Al-Aini University Hospital, Cairo, Egypt. All the patients had skin phototype III, IV or V and none of them had received phototherapy before.
Topical and systemic therapies were discontinued 2 months prior to the initiation of phototherapy.
Inclusion criteria included patients older than 10 years diagnosed with photo-responsive dermatoses including Stages Ia, Ib or IIa of mycosis fungoides, psoriasis vulgaris affecting more than 30% of body surface area and patients with non-segmental vitiligo affecting 30–60% of body surface area.
Pregnant or lactating women, patients with photosensitive disorders, arthropathic, pustular or erythrodermic psoriasis and patients with any absolute or relative contraindication to phototherapy were excluded. In the control population, individuals with habitual or occupational heavy sun exposure were also excluded.
The patients were randomly divided into three groups. Allocation concealment was done using opaque sealed envelopes.
Each of the three groups included 21 patients (7 patients with each disease). Group 1 received narrowband ultraviolet-B, Group 2 received psoralen plus ultraviolet-A and Group 3 received ultraviolet-A phototherapy, in a dose of 10–5 J/cm [2]. Group 4 (control group) included 21 healthy individuals having no dermatological diseases.
Methodology
The study was approved by the institute's research ethical committee. Written informed consent was obtained from each patient and control before inclusion.
A detailed history was elicited from each patient with special emphasis on sun exposure, any special habits and relevant concomitant or previous medications.
All patients were subjected to a complete skin examination and a panel of investigations according to the guidelines for each disease and the line of phototherapy used.
Phototherapy equipment and regimens
- Narrowband ultraviolet-B was delivered by UV100 Waldmann cabins, equipped with 16 ultraviolet-B lamps emitting a radiation of 310–315 nm with a peak at 311 nm. Initial dosage and subsequent increments were dependent on the minimal erythema dose [7]
- Psoralen plus ultraviolet-A and broadband ultraviolet-A were delivered by UV1000 Waldmann cabins, equipped with 26 ultraviolet-A lamps emitting a radiation spectrum of 315–400 nm with a peak of 365 nm and emission power of 10 mw/cm [2]
- Psoralen plus ultraviolet-A treated patients received 8-methoxy psoralen, 2 h before sessions in a dose of 0.5–0.7 mg/kg. The starting dose and subsequent increments were dependent on the skin-type and the dermatoses. For ultraviolet-A treated patients, the dose was fixed at 10 J/cm[2] for mycosis fungoides and psoriasis patients, and at 15 J/cm[2] for vitiligo patients.[8],[9],[10],[11],[12]
Treatment was continued until more than 80% clearance of the lesions was achieved.
Skin biopsies
Two punch biopsies of 5 mm each were taken under local anesthesia from the the lesional sun-protected areas in 63 patients one before initiation of phototherapy and one after the last session. One biopsy was taken from the sun-protected skin of each of the 21 control participants.
Preparation of skin biopsies
The skin biopsy specimen was rinsed in ice-cold phosphate buffered saline (pH 7.0) to remove excess blood and was weighed before homogenization. Each specimen was minced into small pieces and homogenized in 300 µl phosphate-buffered saline on ice. The resulting suspension was subjected to ultrasonication to break the cell membranes. The homogenate was centrifuged for 15 min at 1500 g and the supernatant was separated for determination of 8-hydroxy-2'-deoxyguanosine by an enzyme-linked immunosorbent assay kit.
Principle of the experiment
8-hydroxy-2'-deoxyguanosine enzyme-linked immunosorbent assay kit uses a quantitative sandwich enzyme immunoassay technique and is supplied by blue gene Biotech (Shanghai, China). According to the manual of instructions provided with the kit, the microtiter plate has been pre-coated with an 8-hydroxy-2'-deoxyguanosine monoclonal antibody. When standards or supernatant of samples are added to the microtiter plate wells, 8-hydroxy-2'-deoxyguanosine if present, will combine to the antibody precoated wells. A standardized preparation of horseradish peroxidase-conjugated polyclonal antibody, specific for 8-hydroxy-2'-deoxyguanosine is added to the previous wells to “sandwich” the 8-hydroxy-2'-deoxyguanosine immobilized on the plate. After the recommended incubation, the wells are thoroughly washed to remove the unbound components. Then, substrate solutions are added to wells. The enzyme (horseradish peroxidase) and substrate are allowed to react. Wells that contain 8-hydroxy-2'-deoxyguanosine and enzyme-conjugated antibody will change color. The enzyme-substrate reaction is terminated by the addition of sulfuric acid (stop solution) and the optical density is measured spectrophotometrically at a wavelength of 450 nm. A standard curve is plotted relating the optical density to the concentration of standards. The 8-hydroxy-2'-deoxyguanosine concentration in each sample is determined from the standard curve plotted.
Results
Summary of the patients' data is represented in [Table - 1].
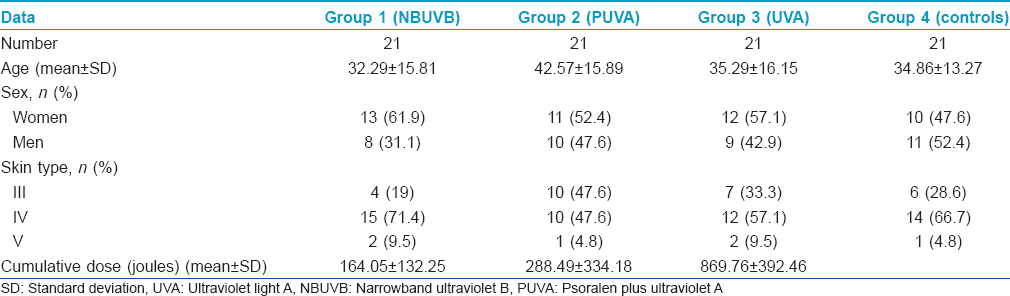
To ensure that the diseases have no influence on the levels of 8-oxoguanine either before or after phototherapy, comparison of 8-oxoguanine levels before and after phototherapy in different diseases (regardless of the type of phototherapy) versus 8-oxoguanine level in controls was done and it showed no statistically significant difference (P > 0.05) [Table - 2].
A strongly significant positive correlation (P < 0.001) was found between 8-oxoguanine level in patients before and after treatment by narrowband ultraviolet-B, psoralen plus ultraviolet-A and ultraviolet-A (r = 0.833, 0.724 and 0.803) respectively, indicating that the pre-treatment baseline 8-oxoguanine level in each patient showed a relatively constant rise after receiving any of the three aforementioned phototherapies. However, this rise was not significant, when compared to controls; there was no statistically significant difference (P: 0.08) on comparing 8-oxoguanine levels in patients before and after treatment by narrowband ultraviolet-B, psoralen plus ultraviolet-A and ultraviolet-A versus 8-oxoguanine level in controls.
Post-treatment 8-oxoguanine levels were statistically significantly higher than pre-treatment levels in patients treated with the three lines of phototherapy (regardless of the disease) or in patients with different diseases (P = <0.001, =0.018, respectively) [Table - 3].
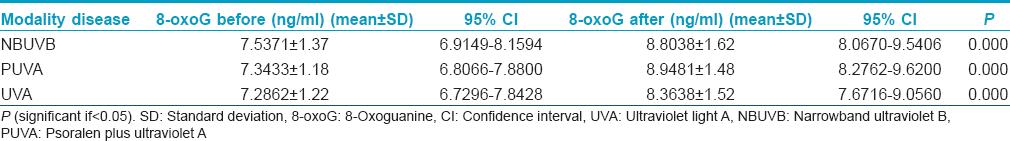
Only for psoralen plus ultraviolet-A treated patients, a weakly significant positive correlation (P = 0.038, r = 0.455) was found between cumulative dose and 8-oxoguanine level after treatment, a finding that shows that every patient receiving psoralen plus ultraviolet-A with a certain cumulative dose will have an increase in 8-oxoguanine level although it is still within the safe limit (compared with 8-oxoguanine level in controls).
Age, extent of disease, the number of sessions and duration of treatment did not correlate with 8-oxoguanine level in patients receiving narrowband ultraviolet-B, psoralen plus ultraviolet-A and ultraviolet-A.
There was no statistically significant difference (P > 0.728) in 8-oxoguanine levels between different skin types among study patients while a significantly lower level of 8-oxoguanine was noted in controls with skin type IV (P = 0.003) compared to those with skin type III.
Discussion
Despite the excessive worldwide fear of photocarcinogenesis, the situation seems to be quite different when it comes to the dark-skinned population. The elaborate mechanisms behind how ultraviolet radiation induces skin cancer is still a matter of interest, but the four events that are proven to be implicated include DNA damage with subsequent gene mutation, disturbed apoptosis, immunosuppression and inflammation.[13] On the other hand, the increased susceptibility of the basal layers of epidermis (from which most skin cancers arise) to ultraviolet-A induced mutations is probably explained by the lower levels of the DNA repair enzyme oxoguanine DNA glycosylase 1 in the basal cells.[14]
Skin phototype is a cardinal effector in the potentiation of or protection against photocarcinogenesis. The fact that darker skinned groups show a lower incidence of cutaneous malignancies emphasizes the photoprotective role of increased epidermal melanin.[15] The ability of the larger, more melanized melanosomes in the epidermis of dark skin to absorb and scatter more light energy than the smaller, less melanized melanosomes of white skin, coupled with decreased DNA damage and greater ultraviolet-induced apoptosis suggest that pigmented epidermis is an efficient ultraviolet filter and explains why individuals with higher skin phototypes possess a lower risk of photocarcinogenesis.[16],[17],[18]
Our study revealed that the different skin phototypes of participants had no influence on the levels of 8-oxoguanine either before or after treatment by any of the phototherapeutic modalities, as proved by the absence of a statistically significant difference when comparing 8-oxoguanine levels in all participants. However, when the same comparison was done in the trunk and chest sun-protected skin, it showed significantly lower levels of 8-oxoguanine in skin type IV. We were not able to find other studies addressing this issue.
In general, ultraviolet-A has been shown to be more carcinogenic than ultraviolet-B being not only immunosuppressive but also mutagenic for basal keratinocytes. The fact that ultraviolet-A in contrast to ultraviolet-B is only weakly absorbed by DNA itself suggests that the indirect pathway involving generation of reactive oxygen species is one way by which ultraviolet-A induces DNA damage.[19],[20],[21]
Practically speaking, narrowband ultraviolet-B seems to be a safe phototherapeutic modality for patients with skin phototypes III–V. A retrospective Korean study found no increase in the incidence of skin cancer in 445 patients with various dermatoses treated with narrowband ultraviolet-B in the period between March 1998 and June 2009 when compared to a control Korean population.[22] On the contrary, in a study done on mice by Kunisada et al., narrowband ultraviolet-B was found to be more carcinogenic than broad band ultraviolet-B due to the formation of cyclobutane pyrimidine dimers.[23]
In contrast to our results, many review articles have described ultraviolet-A to be carcinogenic.[24],[25],[26],[27],[28] The carcinogenic potential of ultraviolet-A was assessed in some studies by the formation of ultraviolet-A-induced DNA double-strand breaks (following a dose of 200 kJ/m [2] of ultraviolet-A) using the Comet assay in human keratinocytes and primary human skin fibroblasts or the formation of cyclobutane pyrimidine dimers in fibroblasts, keratinocytes, and melanocytes or by the activation of the p38α signaling pathway, which is a mitogen-activated protein kinase involved in ultraviolet-induced apoptosis, following a dose of 60 kJ ultraviolet-A in mice.[29],[30],[31] However, these studies were done in vitro on cell cultures and did not compare their results with normal skin with an average exposure to ultraviolet radiation in therapeutic doses.
As far as the carcinogenic potential of psoralen plus ultraviolet-A is concerned, this was assessed in some studies by inducing mutations in the p53 tumor suppressor gene in yeasts incubated with 8-methoxypsoralen and ultraviolet-A irradiated in-vitro by 5 or 10 kJ/m [2] (0.5–1 J/cm [2]).[32] It was also assessed on a human fibroblast cell culture that was irradiated with 30 kJ/m [2] (3 J/cm [2]) ultraviolet-A via formation of DNA monoadducts and interstrand crosslinks.[33]
Parameters associated with increased risk of carcinogenic potential could be related to patient characteristics, underlying disease, phototherapy type, ultraviolet dose or duration. Therefore, in vivo studies defining risk parameters in different populations are essential and are still lacking.
In our study, we did not find a significant difference in 8-oxoguanine levels between controls and the patients (of the three included dermatoses) before and after treatment by the three modalities. Accordingly, we concluded that different phototherapeutic modalities do not seem to have significant carcinogenic potential within the therapeutic doses in dark skinned individuals. This finding also suggests that the type of disease is not a determinant in causing increased ultraviolet-induced carcinogenic potential. We could also find no correlation between 8-oxoguanine levels in the patients and their age, disease extent, number of ultraviolet sessions and duration of phototherapy.
The only finding which raised our concern was the weakly significant positive correlation between the cumulative doses of psoralen plus ultraviolet-A and 8-oxoguanine levels after treatment. We therefore emphasize that the use of psoralen plus ultraviolet-A should be coupled with caution as regards patient selection and dose adjustment to minimize the cumulative dose and the subsequent possible carcinogenic risk.
In contrast to our results, a study demonstrated significantly increased 8-oxoguanine levels in 20 vitiligo patients, in comparison to 10 healthy controls.[34] A literature review showed no studies measuring the levels of 8-oxoguanine in mycosis fungoides or psoriasis.
Finally, we conclude that although therapeutic doses of ultraviolet radiation appear to pose no increased risk of carcinogenesis in the darker skinned population, the cumulative doses of all phototherapeutic modalities, particularly that of psoralen plus ultraviolet-A should be carefully kept to a minimum. This can be achieved by introducing individualized combinations or rotational therapeutic strategies as early as possible instead of focusing on phototherapy as a sole treatment option. Furthermore, more effective phototherapy regimens should be established that determine maximum number of sessions for each disease, after which cessation of phototherapy becomes necessary, regardless of the response. Surely, longer studies with higher cumulative doses will help in either confirming or negating our findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Young AR, Walker SL. Acute and chronic effects of ultraviolet radiation on the skin. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. New York: McGraw-Hill Medical; 2008. p. 809-15.
[Google Scholar]
|
| 2. |
Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol 2005;44:1016-21.
[Google Scholar]
|
| 3. |
Walker D, Jacobe H. Phototherapy in the age of biologics. Semin Cutan Med Surg 2011;30:190-8.
[Google Scholar]
|
| 4. |
Syed ZU, Hamzavi IH. Role of phototherapy in patients with skin of color. Semin Cutan Med Surg 2011;30:184-9.
[Google Scholar]
|
| 5. |
Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA). A meta-analysis. Arch Dermatol 1998;134:1582-5.
[Google Scholar]
|
| 6. |
Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res 2007;67:3468-74.
[Google Scholar]
|
| 7. |
Gordon PM, Saunders PJ, Diffey BL, Farr PM. Phototesting prior to narrowband (TL-01) ultraviolet B phototherapy. Br J Dermatol 1998;139:811-4.
[Google Scholar]
|
| 8. |
Melski JW, Tanenbaum L, Parrish JA, Fitzpatrick TB, Bleich HL. Oral methoxsalen photochemotherapy for the treatment of psoriasis: A cooperative clinical trial. J Invest Dermatol 1977;68:328-35.
[Google Scholar]
|
| 9. |
El Mofty M, Ramadan S, Fawzy MM, Hegazy RA, Sayed S. Broad band UVA: A possible reliable alternative to PUVA in the treatment of early-stage mycosis fungoides. Photodermatol Photoimmunol Photomed 2012;28:274-7.
[Google Scholar]
|
| 10. |
El-Mofty M, Mostafa WZ, Yousef R, Abdel Halim MR, El Hawary M, Abdel Kader H, et al. Broadband ultraviolet A in the treatment of psoriasis vulgaris: A randomized controlled trial. Int J Dermatol 2014;53:1157-64.
[Google Scholar]
|
| 11. |
El-Mofty M, Mostafa W, Youssef R, El-Fangary M, Elramly AZ, Mahgoub D, et al. Ultraviolet A in vitiligo. Photodermatol Photoimmunol Photomed 2006;22:214-6.
[Google Scholar]
|
| 12. |
El-Mofty M, Mostafa W, Youssef R, El-Fangary M, El-Ramly A, Mahgoub D, et al. BB-UVA vs. NB-UVB in the treatment of vitiligo: A randomized controlled clinical study (single blinded). Photodermatol Photoimmunol Photomed 2013;29:239-46.
[Google Scholar]
|
| 13. |
Norval M, Halliday GM. The consequences of UV-induced immunosuppression for human health. Photochem Photobiol 2011;87:965-77.
[Google Scholar]
|
| 14. |
Javeri A, Huang XX, Bernerd F, Mason RS, Halliday GM. Human 8-oxoguanine-DNA glycosylase 1 protein and gene are expressed more abundantly in the superficial than basal layer of human epidermis. DNA Repair (Amst) 2008;7:1542-50.
[Google Scholar]
|
| 15. |
Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. Faseb J 2003;17:1177-9.
[Google Scholar]
|
| 16. |
Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol 2010;49:978-86.
[Google Scholar]
|
| 17. |
Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, et al. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. Faseb J 2006;20:1486-8.
[Google Scholar]
|
| 18. |
Yamaguchi Y, Beer JZ, Hearing VJ. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: Factors influencing the incidence of skin cancer. Arch Dermatol Res 2008;300 Suppl 1:S43-50.
[Google Scholar]
|
| 19. |
Sutherland JC, Griffin KP. Absorption spectrum of DNA for wavelengths greater than 300 nm. Radiat Res 1981;86:399-409.
[Google Scholar]
|
| 20. |
Rünger TM, Kappes UP. Mechanisms of mutation formation with long-wave ultraviolet light (UVA). Photodermatol Photoimmunol Photomed 2008;24:2-10.
[Google Scholar]
|
| 21. |
Ridley AJ, Whiteside JR, McMillan TJ, Allinson SL. Cellular and sub-cellular responses to UVA in relation to carcinogenesis. Int J Radiat Biol 2009;85:177-95.
[Google Scholar]
|
| 22. |
Jo SJ, Kwon HH, Choi MR, Youn JI. No evidence for increased skin cancer risk in Koreans with skin phototypes III-V treated with narrowband UVB phototherapy. Acta Derm Venereol 2011;91:40-3.
[Google Scholar]
|
| 23. |
Kunisada M, Kumimoto H, Ishizaki K, Sakumi K, Nakabeppu Y, Nishigori C. Narrow-band UVB induces more carcinogenic skin tumors than broad-band UVB through the formation of cyclobutane pyrimidine dimer. J Invest Dermatol 2007;127:2865-71.
[Google Scholar]
|
| 24. |
El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. A review of human carcinogens – part D: Radiation. Lancet Oncol 2009;10:751-2.
[Google Scholar]
|
| 25. |
Ikehata H, Ono T. The mechanisms of UV mutagenesis. J Radiat Res 2011;52:115-25.
[Google Scholar]
|
| 26. |
Mitchell D. Melanoma back in the UVA spotlight. Pigment Cell Melanoma Res 2012;25:540-1.
[Google Scholar]
|
| 27. |
Moan J, Baturaite Z, Porojnicu AC, Dahlback A, Juzeniene A. UVA, UVB and incidence of cutaneous malignant melanoma in Norway and Sweden. Photochem Photobiol Sci 2012;11:191-8.
[Google Scholar]
|
| 28. |
Chen AC, Halliday GM, Damian DL. Non-melanoma skin cancer: Carcinogenesis and chemoprevention. Pathology 2013;45:331-41.
[Google Scholar]
|
| 29. |
Greinert R, Volkmer B, Henning S, Breitbart EW, Greulich KO, Cardoso MC, et al. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res 2012;40:10263-73.
[Google Scholar]
|
| 30. |
Kim SI, Jin SG, Pfeifer GP. Formation of cyclobutane pyrimidine dimers at dipyrimidines containing 5-hydroxymethylcytosine. Photochem Photobiol Sci 2013;12:1409-15.
[Google Scholar]
|
| 31. |
Liu K, Yu D, Cho YY, Bode AM, Ma W, Yao K, et al. Sunlight UV-induced skin cancer relies upon activation of the p38α signaling pathway. Cancer Res 2013;73:2181-8.
[Google Scholar]
|
| 32. |
Monti P, Inga A, Aprile A, Campomenosi P, Menichini P, Ottaggio L, et al. p53 mutations experimentally induced by 8-methoxypsoralen plus UVA (PUVA) differ from those found in human skin cancers in PUVA-treated patients. Mutagenesis 2000;15:127-32.
[Google Scholar]
|
| 33. |
Derheimer FA, Hicks JK, Paulsen MT, Canman CE, Ljungman M. Psoralen-induced DNA interstrand cross-links block transcription and induce p53 in an ataxia-telangiectasia and rad3-related-dependent manner. Mol Pharmacol 2009;75:599-607.
[Google Scholar]
|
| 34. |
Salem MM, Shalbaf M, Gibbons NC, Chavan B, Thornton JM, Schallreuter KU. Enhanced DNA binding capacity on up-regulated epidermal wild-type p53 in vitiligo by H2O2-mediated oxidation: A possible repair mechanism for DNA damage. Faseb J 2009;23:3790-807.
[Google Scholar]
|
Fulltext Views
1,590
PDF downloads
1,009





