Translate this page into:
PLACK syndrome associated with alopecia areata and a novel homozygous base pair insertion in exon 18 of CAST gene
Corresponding author: Dr. Anza Khader, Department of Dermatology, Government Medical College, Kozhikode, Kerala, India. anzashaan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vidya AS, Khader A, Devi K, Archana GA, Reeshma J, Reshma NJ. PLACK syndrome associated with alopecia areata and a novel homozygous base pair insertion in exon 18 of CAST gene. Indian J Dermatol Venereol Leprol. 2023;90:102-5. doi: 10.25259/IJDVL_1138_2021
Dear Editor,
Peeling skin syndromes (PSS) are rare autosomal recessive genodermatoses characterised by superficial painless shedding of stratum corneum.1,2 Traupe classified PSS into Type A and B in which the former presents without skin blistering and the latter is a form of exfoliative ichthyosis.3 Lin et al in 2015 first reported four cases with generalised peeling, leukonychia, acral punctate keratoses, cheilitis, knuckle pads and proposed an acronym PLACK syndrome.2
An 11-year-old boy born to nonconsanguinous parents presented with a history of recurrent bullae and peeling of skin subsiding with hyperpigmentation since 3 months of age. Peeling of skin with or without bullae developed spontaneously or following trauma. It was generalised till 7 years of age and later remained confined to extremities. The child also had recurrent episodes of patchy hair loss in the scalp. The child had no significant systemic illnesses. There was no history of similar lesions in the family.
Clinical examination revealed generalised xerosis, peeling of skin over dorsae of hands and feet, forearm, elbows, knees and lower legs, punctate palmoplantar keratoderma, hyperkeratotic papules over knuckles and dorsae of feet and multiple hyperkeratotic follicular papules over both lower limbs. Though all nails showed proximal leukonychia and distal onycholysis, partial nail dystrophy was seen only on toe nails [Figures 1a, 1b, 1c and 1d]. A single patch of non scarring alopecia over the vertex of scalp was noted and the dermoscopy of this patch revealed yellow dots, black dots, short vellus hair and exclamation mark sign [Figures 2a and 2b].
Skin biopsy from peeled area showed subcorneal blister and from the hyperkeratotic papule showed hyperkeratosis and acanthosis [Figures 3a and 3b].
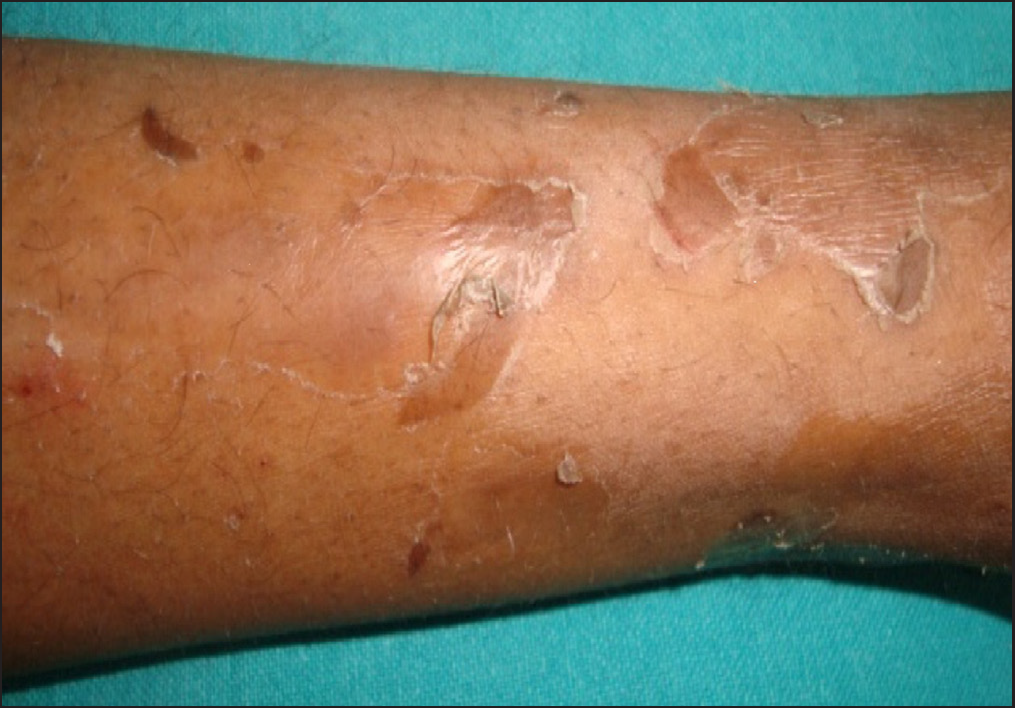
- Peeling of skin over the extensor aspect of left leg.
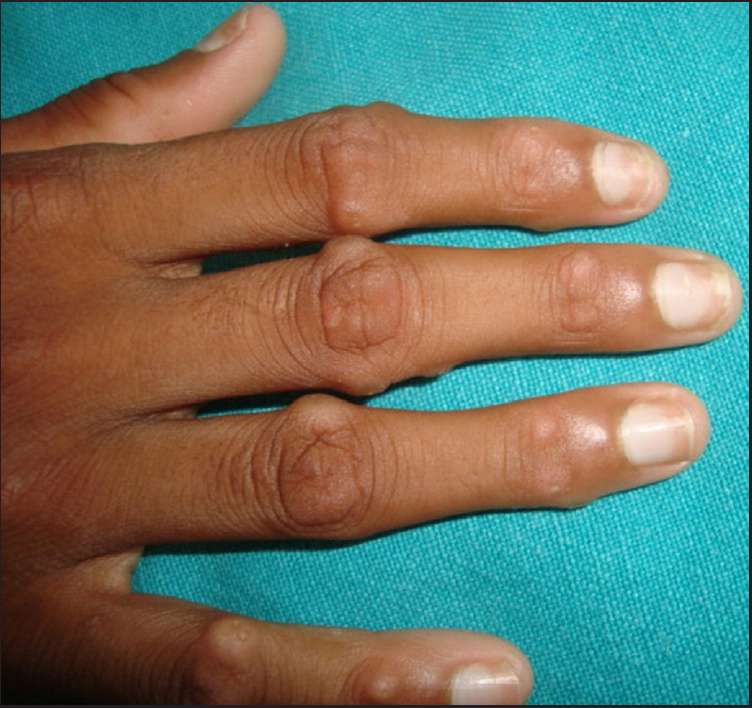
- Hyperkeratotic papules over knuckles and proximal leukonychia.
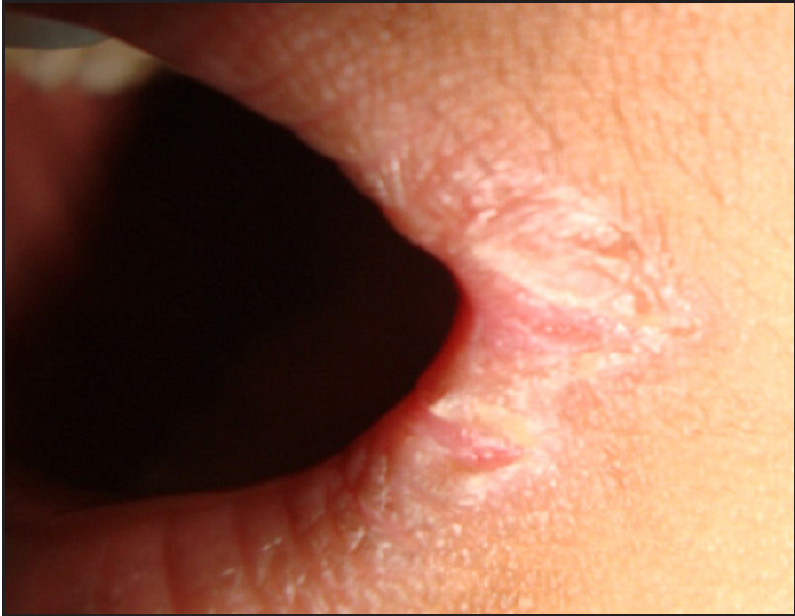
- Angular cheilitis.
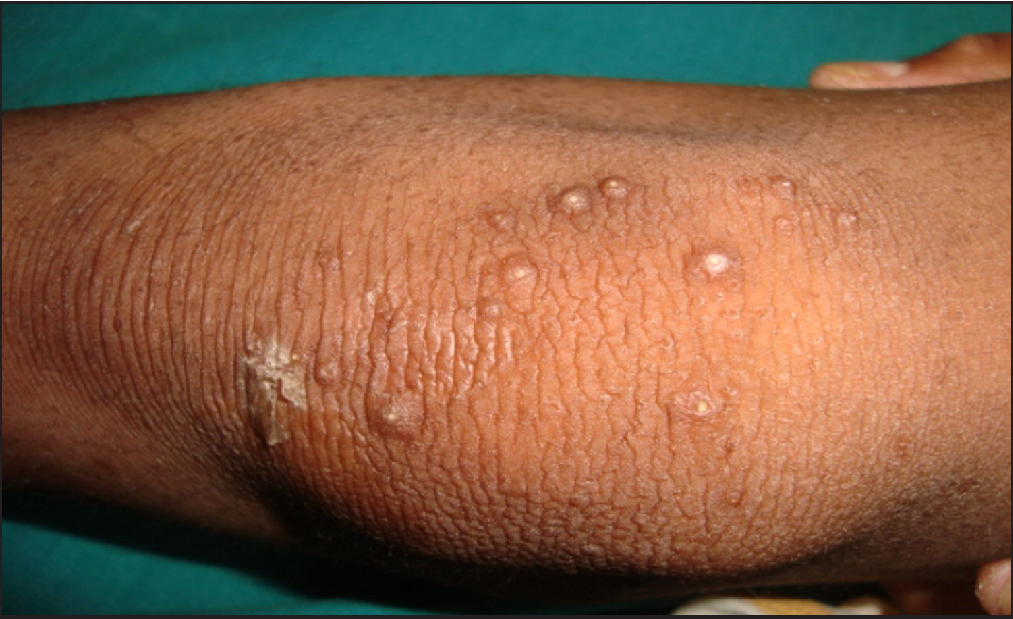
- Multiple hyperkeratotic follicular papules overthe right knee joint.
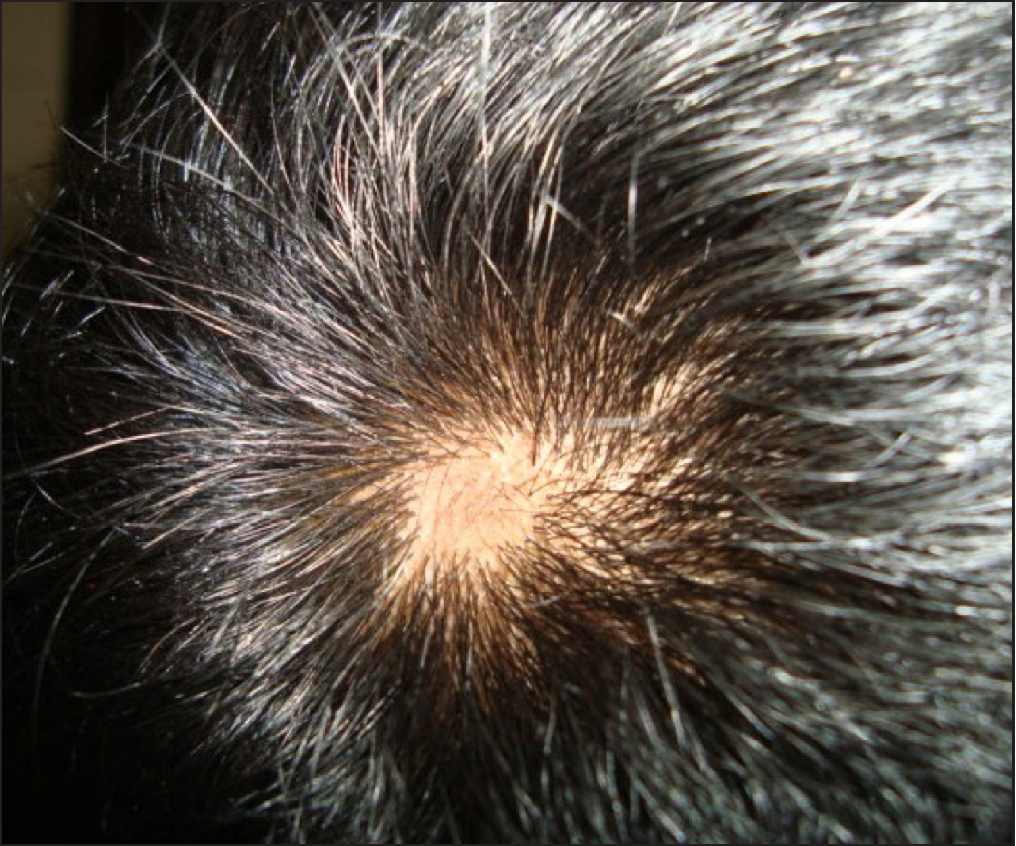
- A patchy area of hair loss over the vertex of scalp.
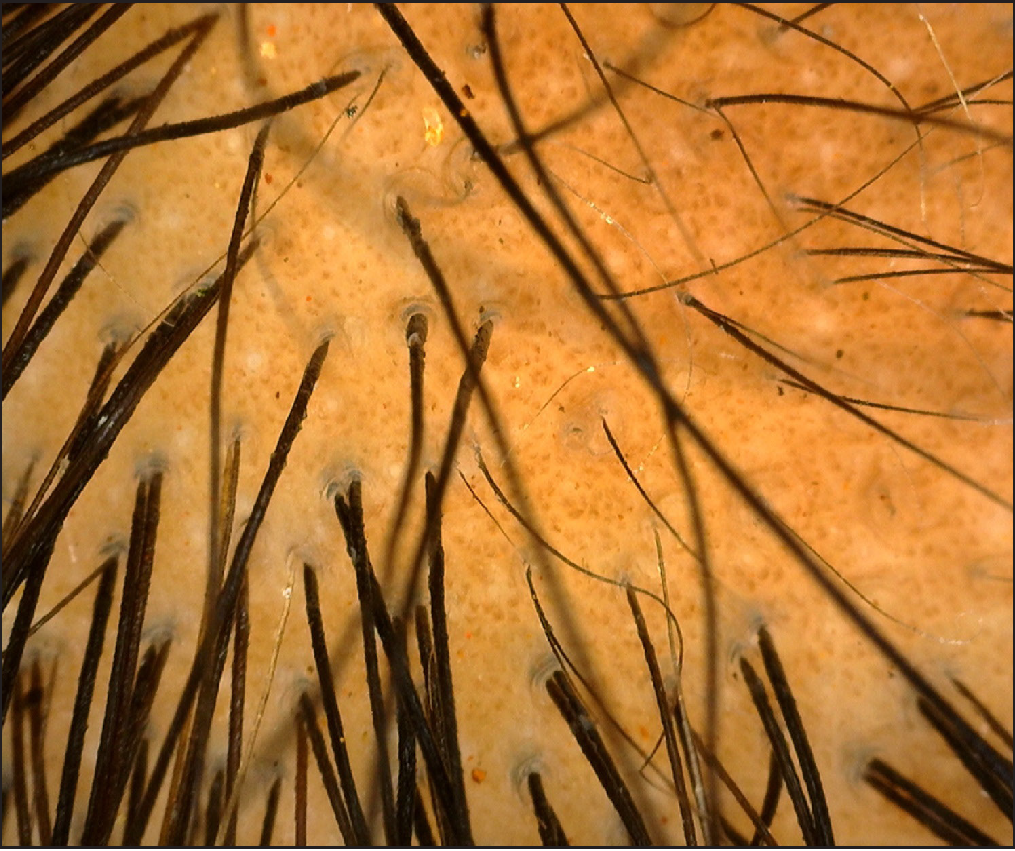
- Scalp dermoscopy showing yellow dots, black dots, short vellus hairs and exclamation mark sign (Dino-Lite premier AM4113T, 200X, polarized).
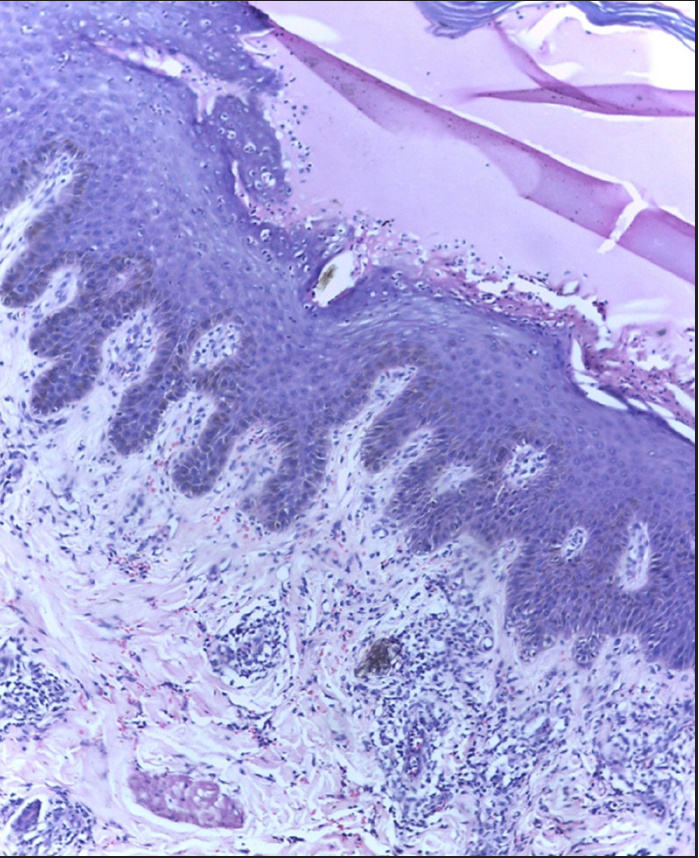
- Skin biopsy from peeled area showing subcorneal separation with neutrophils, acanthosis and perivascular neutrophilic infiltrate (H&E, 400x).

- Skin biopsy from hyperkeratotic papule showing hyperkeratosis, irregular acanthosis, perifollicular and perivascular neutrophilic infiltration (H&E, 400x).
Genomic DNA was isolated from the patient’s blood sample (Qiagen). The specific exon regions from CAST gene were PCR amplified and sequenced using Sanger sequencer (ABI 3500, Thermo Scientific). The chromatogram was analysed for variants but all the previously reported mutations were absent in our patient.
Further whole exome sequencing and subsequent analysis of genomic DNA was performed (MedGenome Labs Ltd).A homozygous single base pair insertion in exon 18 of the CAST gene (chr5:g.96748570_96748571insT; Depth: 33x) encoding calpastatin that resulted in a frameshift mutation and premature truncation of the protein at codon 441 (p.Glu441Ter; ENST00000510756.5) was detected. As the patient had alopecia areata we also looked for the variant of PTPN22 (Protein tyrosine phosphatase non-receptor type 22) encoding Lyp phosphatase (Lyp R620W) and this was detected while doing whole exome sequencing.
PLACK syndrome is an autosomal recessive condition with a prevalence of 1:10,00,000. The onset occurs in infancy and does not affect the life expectancy of the individual.2
PLACK syndrome is characterised by superficial blisters predominantly over the extremities, either of spontaneous onset or induced by trauma. Other features include asymptomatic peeling of skin, generalised xerosis, fissuring of lips and perioral region, white nails and keratotic papules over knuckles and dorsa of hands and feet.2
Six mutations reported till date are loss of function mutations in c.607dup (p.Ile203Asnfs*8), c.424A > T (p.Lys142*), c.1750delG (p.Val584Trpfs*37), c.461dupGCAT (p.Ser154Cysfs*6), c.544G > T (p.Glu182*) and (c.507_508insA).2,4–6 To the best of our knowledge, ours is probably the seventh case and the first from India which is associated with a novel homozygous mutation in the CAST gene and an unreported clinical association of alopecia areata.
CAST gene encodes calpastatin, a specific inhibitor of calpain, an intracellular cysteine protease. Calpains which are expressed normally in human skin are activated by calcium ions and play an important role in cell proliferation, migration, apoptosis, cell survival, epidermal differentiation, cornification and keratinocyte apoptosis.5–7 Mutation in Cast gene leads to decreased calpastatin which results in increased activity of calpains and impairs intracellular adhesion leading to peeling of skin and also affects cell cycle regulation and keratinocyte apoptosis causing hyperkeratosis.5
The gene encoding for Protein tyrosine phosphatase non-receptor type 22 (PTPN22) is exclusively expressed in immune cells. Its non synonymous C1858T substitution in codon 620 (LypR620W) is considered as a risk factor in many autoimmune diseases including rheumatoid arthritis, systemic lupus erythematosus and thyroid diseases.8,9 This LypR620W variant with arginine to tryptophan substitution causes Lyp/Pep (Lymphoidphosphatase/PEST–enriched phosphatase) more susceptible to calpain and proteosome mediated cleavage and degradation. The increased activity of calpains as in our case lowers Lyp/Pep levels and decreases the inhibitory effects on immunological activation result in many autoimmune disorders including alopecia areata.8–11 We report the seventh case of PLACK syndrome with a new variant mutation in CAST gene and a clinical association of alopecia areata hitherto unreported in literature. The CAST gene mutation and its effects on calpain calpastatin system and calpain mediated degradation of Lyp/Pep resulting in lymphocyte and dendritic cell hyper responsiveness might have resulted in alopecia areata in our case. We would like to propose that the individuals with PLACK syndrome may present with various autoimmune diseases if there is associated Lyp/Pep polymorphism although the direct effect of CAST gene on Lyp/Pep requires further studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A case of late-onset peeling skin syndrome likely triggeredby irritation. Ann Dermatol. 2017;29:119-20.
- [CrossRef] [PubMed] [Google Scholar]
- Loss-of-Function Mutations in CAST Cause Peeling Skin, Leukonychia, Acral Punctate Keratoses, Cheilitis, and Knuckle Pads. Am J Hum Genet. 2015;96:440-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peeling‐skin syndrome: Clinical and morphological evidence for two types. In: Traupe H, ed. The Ichthyoses: a guide to clinical diagnosis, genetic counselling, and therapy. Berlin: Springer; 1989. p. :207-10.
- [Google Scholar]
- PLACK syndrome resulting from a new homozygous insertion mutation in CAST. J Dermatol Sci. 2017;88:256-8.
- [CrossRef] [PubMed] [Google Scholar]
- A novel homozygous nonsense mutation in CAST associated with PLACK syndrome. Cell Tissue Res. 2019;378:267-77.
- [CrossRef] [PubMed] [Google Scholar]
- PLACK syndrome shows remarkable phenotypic homogeneity. Clin Exp Dermatol. 2019;44:580-3.
- [PubMed] [PubMed Central] [Google Scholar]
- Role of Calpain in Pathogenesis of Human Disease Processes. J Nat Sci. 2016;2:218.
- [PubMed] [PubMed Central] [Google Scholar]
- Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol. 2014;32:83-119.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association between PTPN22 C1858T polymorphism and alopecia areata risk. Exp Ther Med 2015:1953-58.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902-7.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of calpain-calpastatin system (CCS) in human T cell activation. Oncotarget. 2016;47:76479-95.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





