Translate this page into:
Polycystic ovarian syndrome
2 Department of Endocrinology, P. D. Hinduja National Hospital, Mahim, Mumbai, India
Correspondence Address:
Nina Madnani
Dr. Nina Madnani, Department of Dermatology, P. D. Hinduja National Hospital, Veer Savarkar Marg, Mahim, Mumbai - 400 016
India
| How to cite this article: Madnani N, Khan K, Chauhan P, Parmar G. Polycystic ovarian syndrome. Indian J Dermatol Venereol Leprol 2013;79:310-321 |
Abstract
Polycystic ovarian syndrome (PCOS) is a "multispeciality" disorder suspected in patients with irregular menses and clinical signs of hyperandrogenism such as acne, seborrhoea, hirsutism, irregular menses, infertility, and alopecia. Recently, PCOS has been associated with the metabolic syndrome. Patients may develop obesity, insulin resistance, acanthosis nigricans, Type 2 diabetes, dyslipidemias, hypertension, non-alcoholic liver disease, and obstructive sleep apnoea. Good clinical examination with hematological and radiological investigations is required for clinical evaluation. Management is a combined effort involving a dermatologist, endocrinologist, gynecologist, and nutritionist. Morbidity in addition includes a low "self image" and poor quality of life. Long term medications and lifestyle changes are essential for a successful outcome. This article focuses on understanding the normal and abnormal endocrine functions involved in the pathogenesis of PCOS. Proper diagnosis and management of the patient is discussed.Introduction
In 1935, Stein and Leventhal reported a series of 7 women who presented with oligo/amenorrhoea, hirsutism, obesity, infertility, and bilateral polycystic ovaries (Stein-Leventhal syndrome). [1] Bilateral wedge resection of the enlarged ovaries was therapeutic in normalizing the menses and fertility of the women studied. They concluded that there was a primary ovarian defect, and the disorder was known as polycystic ovarian disease (PCOD). Today, PCOD has been associated with various metabolic disorders, and is now known as polycystic ovarian syndrome (PCOS). Following terminologies are important for better understanding of the clinical syndrome:
- Hyperandrogenemia is the presence of abnormally high amounts of androgens detectable in the blood circulation.
- Hyperandrogenism is the clinical manifestation of excessive androgen production/secretion, characterized by oligo/amenorrhoea, infertility, acne, seborrhoea, hirsutism, and alopecia.
- Cutaneous hyperandrogenism is considered when there is no documented evidence of hyperandrogenemia but the clinical signs of hyperandrogenism are present.
- Hypertrichosis is the presence of excessive hair in non-androgen dependent areas.
- Hirsutism is the presence of excess and coarse terminal hairs in a male-pattern distribution, such as upper lip, chin, cheeks, shoulders, abdomen, lower back and inner thighs.
- Polycystic ovarian disease (PCOD) was described by Stein and Leventhal [1] in women with oligo/amenorrhoea, hirsutism, obesity, infertility and bilateral polycystic ovaries, managed with wedge ovarian resection.
- Polycystic ovarian syndrome (PCOS) is PCOD + metabolic disease.
PCOS is a common disorder with an incidence varying from 5% to 10%. It is observed in women of child-bearing age across all cultures, and ethnicities. [2] A prospective study of Indian adolescents reported an incidence of 9.13%. [3]
The common features of PCOS are irregular or anovulatory cycles with signs of hyperandrogenism like acne, seborrhoea, hirsutism, alopecia, frank virilization, and with polycystic ovaries on pelvic sonography. Recently, PCOS has been associated with obesity, insulin-resistance (IR) and a risk of developing Type 2 diabetes mellitus (T2DM). [4] The metabolic and reproductive abnormalities have predisposed women to develop infertility and endometrial cancer, necessitating early diagnosis and appropriate treatment. [5] A better understanding of the complexities involved in PCOS necessitates the understanding of what is "normal ovulation".
- A normal ovulatory cycle is maintained due to a complex interplay of hormones secreted from the pituitary, hypothalamus, adrenals, and ovaries. Up-regulation or down-regulation of these hormones is carried out by an efficient feed-back mechanism to maintain equilibrium [Figure - 1]. The hypothalamus secretes gonadotropin releasing hormone (GnRH) in a fixed, pulsatile manner. This acts upon the cells of the anterior pituitary to secrete two important hormones, the follicle stimulating hormone (FSH) and the leutinising hormone (LH), both targeting the follicles in the outer cortex of the ovary which are in various stages of development. FSH secretion is regulated by a negative feedback from estradiol, inhibin and progesterone. The inner lining of the ovarian follicles is composed of granulosa cells and theca cells. Receptors for FSH are exclusively present on the granulosa cells. Under the influence of FSH, the follicles are recruited, but only one developing follicle undergoes maturation to become the dominant or graffian follicle. Interestingly, early stages of follicular development up to 2 to 5 mm seem to be independent of the need for FSH. FSH is also essential for induction of aromatase activity within the granulosa cells. LH receptors are expressed by the theca cells of all follicles and the granulosa cells of large pre-ovulatory follicles. It is the stimulation of the latter receptors by the LH surge seen in mid-cycle, which is responsible for ovulation. Estradiol has a positive feedback response for LH release at the level of pituitary. Also, LH plays a vital role in androgen production by the theca cells.
 |
| Figure 1: Feedback mechanism for a normal menstrual cycle |
Ovarian Steroidogenesis
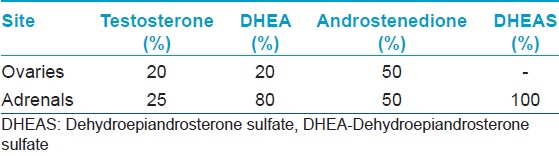
Cholesterol, the precursor for steroid synthesis in the ovaries, undergoes a series of conversions to form androstenedione (enzymes 3β-HSD, 17α-hydroxylase and 17, 20-lyase mediate this conversion). Androstenedione gets converted to testosterone (by 17β-HSD) and to estrone (by aromatase) in the granulosa cells [Figure - 2]. The ovaries produce 25% of circulating testosterone, which is dependent on LH secreted by the anterior pituitary. The ovaries also secrete 50% of the androstenedione and 20% of dehydroepiandrosterone (DHEA) [Table - 1]. Testosterone is used as a marker of ovarian androgen secretion.
 |
| Figure 2: Ovarian steroidogenesis: Granulosa cells form the inner lining of the ovarian follicle. The thecal cells abut them and form the outer layer. Granulosa cells are dependent on the diffusing androstenedione from the theca cells for substrate as they are not in direct connection with the circulation (Two cell hypothesis). All steroid hormones are derived from cholesterol. Its entry into the mitochondria is mediated by StAR (Steroidogenic acute regulatory protein) and is the rate limiting step in ovarian steroidogenesis 3 β HSD: Hydroxysteroid dehydrogenenase |
Extra Ovarian Steroidogenesis
The adrenals synthesize androgens from cholesterol, under the influence of adreno corticotropic hormone (ACTH) (secreted by the anterior pituitary), through multiple steps. The adrenal androgens consist of all the dehydroepiandrosterone sulphate (DHEAS) and 80% of the DHEA. The adrenals also secrete 50% of androstenedione and 25% of circulating testosterone. DHEAS and 11-androstenedione are not secreted by the ovaries, and therefore are used as markers of adrenal androgen secretion. Both prolactin and estrogen can affect adrenal androgen production. The peripheral tissues like the skin, liver, and adipose tissue also take part in androgen synthesis by converting the weaker androgens to the more potent ones. Androgen receptor-activation takes place only with testosterone and dihydrotestosterone (DHT). 25% of the circulating testosterone is produced individually by the ovaries and adrenals. The remaining 50% by the peripheral conversion of androstenedione. [6] Only 1% of the androgens produced circulates freely. Of the remaining 99%, 80% is bound to sex hormone binding globulin (SHBG), and 18% to albumin. If SHBG levels are reduced, the amount of free circulating testosterone increases. DHEAS, DHEA and androstenedione are bound to albumin and freely available to tissues. There is no regulatory feedback mechanism in both the adrenals and ovaries in response to androgen production.
Role of Insulin
Insulin plays an important role in the normal ovarian cycle in the following ways: It enhances the amplitude of LH pulses at the level of pituitary, but not their frequency. It modulates the LH-mediated androstenedione production in theca cells and FSH-mediated estradiol production in granulosa cells, probably by increasing ovarian cytochrome P457 enzyme activity. In the liver, increased insulin levels inhibit production of sex hormone binding globulin (SHBG). It inhibits hepatic production of IGFBP-1 which results in increased levels of circulating IGF-1 (Insulin like growth factor 1) levels. It binds to the IGF-1 receptors on the ovary and directly stimulates androgen production. [7]
Evolution of polycystic ovarian syndrome
The key steps in the evolution of PCOS are enumerated below, but not necessarily in chronological order.
Pituitary dysfunction
There is an increase in the frequency and amplitude of GnRH pulse which results in elevated levels of LH. However, whether this alteration in LH secretion is due to an intrinsic defect of the hypothalamus or due to external factors such as loss of negative feedback mechanism from the ovary, or increase in insulin levels, remains an unresolved debate. Excessive secretion of LH has traditionally been considered as a hallmark of classic PCOS. An LH/FSH ratio of more than 2 especially in obese women is significant.
Enhanced ovarian androgen production
Abnormal LH influence on the thecal cells of the ovaries results in overproduction of androgens. However, all patients of PCOS do not have elevated levels of LH but still have elevated levels of androgens. This can be explained on the findings that ovarian thecal cells of women with PCOS have elevated CYP17 gene expression. The encoded enzyme (P450c17) is capable of both, 17-hydroxylation and 17, 20-layse functions which is a key step in ovarian steroidogenesis. [8] Neelson et al., in 2001 showed that the p450c17 and 3β-HSD enzyme activity is disproportionately higher in the thecal cells of PCOS women as compared to normal women. So, the enhanced production of testosterone precursors is the probably the cause of elevated androgen levels.
Development of insulin resistance
Women with PCOS have decreased sensitivity to circulating insulin irrespective of weight (obesity), thus suggesting that this insulin insensitivity is intrinsic to PCOS. [9] This has been attributed to a defect in phosphorylation of tyrosine kinase in the insulin receptor. Normally, tyrosine autophosphorylation increases the insulin - mediated metabolic changes within muscle and adipose cells. Serine phosphorylation inhibits this action. Also, serine phosphorylation has a stimulatory effect on 17,20-lyase enzyme activity in the ovaries which is responsible for production of androstenedione. Up to 50% of women with PCOS have a genetic defect coding for serine phosphorylation of insulin receptors. The resulting increased insulin levels, directly and indirectly, increase androgen production. [10] So conversely, a reduction in serum insulin levels should result in reduction in androgen levels.
Etiopathogenesis
Although the exact etiology is unknown, PCOS is a multi-factorial disease with a strong genetic influence. Various animal models have been used to understand the pathophysiology of PCOS. Each model has its own limitation but rodents are the most widely used. [11] Others include mice, rhesus monkeys, ewes and more recently, sheep. These models have provided evidence that intra-uterine fetal exposure to androgen excess is associated with development of PCOS in later life. [12] Genetic basis of this disease can be seen from studies evaluating the genetic pool of patients with PCOS. Legro and colleagues studied the siblings of 80 patients diagnosed with PCOS. Out of a total of 115 sisters, 46% had evidence of hyperandrogenemia and amongst them, almost half fulfilled the criteria for PCOS. It was also noted that brothers of the patients with PCOS had increased levels of DHEAS when compared with age matched controls. [13] Fathers of adolescent girls have a very high incidence of obesity (94%) and metabolic syndrome (79%). Also, mothers of such patients tend to be obese (54.4%) or overweight (11.4%) with the incidence of metabolic syndrome being 34%. [14]
Clinical Features
The patient seeks a dermatology consultation for one or more complaints like acne, hirsutism, alopecia, acanthosis nigricans, skin tags and occasionally, darkening of complexion with weight gain. If irregular menstrual cycles or primary infertility are the main complaints, the patient may consult a gynaecologist. An endocrinologist may be consulted for hirsutism and the metabolic syndrome. Very rarely do patients present with all the clinical signs and symptoms of PCOS and some may not be forthcoming with information of concurrent treatment from a gynaecologist or an endocrinologist. An alert clinician should be able to link the symptoms together, pointing to a possible underlying defect of hyperandrogenism.
Acne vulgaris
Patients with PCOS complain of inflammatory acne minimally responsive to conventional line of treatment. Even if responsive, lesions promptly recur on stopping treatment, necessitating treatment with oral isotretinoin and/or hormonal therapy. An important feature seen in these patients is the development of multiple closed comedones which rapidly transform into tender, lumpy nodules, distributed in the lower half of face and jaw-line (V distribution) [Figure - 3]. These tend to persist beyond the usual course of 5-7 days. A pre-menstrual flare is also common. Acne lesions may not only be localized to the face, but may also be present on the chest, shoulders and back. Prompt relapse after stopping the treatment, strongly suggests a hormonal basis. Patients may in addition, have a history of irregular periods, and evidence of hirsutism, alopecia, or a positive family history of PCOS. The severity of hirsutism may not be matching the severity of acne, and would be dependent on the balance of activity between the alfa-hydroxy type 2 vis-a-vis type 1. [15]
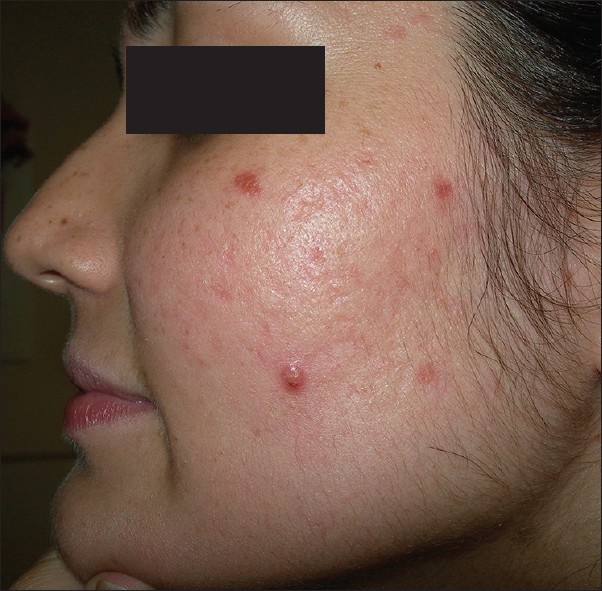 |
| Figure 3: Acne with hirsutism in a patient with PCOS |
Hirsutism
Excessive facial hair is a racial trait for the Indian sub-continent, running within families and especially strong in certain ethnic groups. This should be kept in mind while evaluating patients complaining of excessive facial/body hair. Androgens affect various aspects of follicular activity. Acting via androgen receptors and secretory factors, they increase the growth rate, diameter and melanization of hair in androgen-sensitive areas. It is these thick, coarse, terminal hair, in androgen-dependent areas which are unsightly on a female [16] and point to an underlying hyper-androgenic state [Figure - 4]. Evaluation of the degree of hirsutism is done by adopting a modified Ferriman-Galway score which evaluates 9 body areas on a scale of 1 to 4. The total scores are significant if more than 6 to 8. [15],[17]
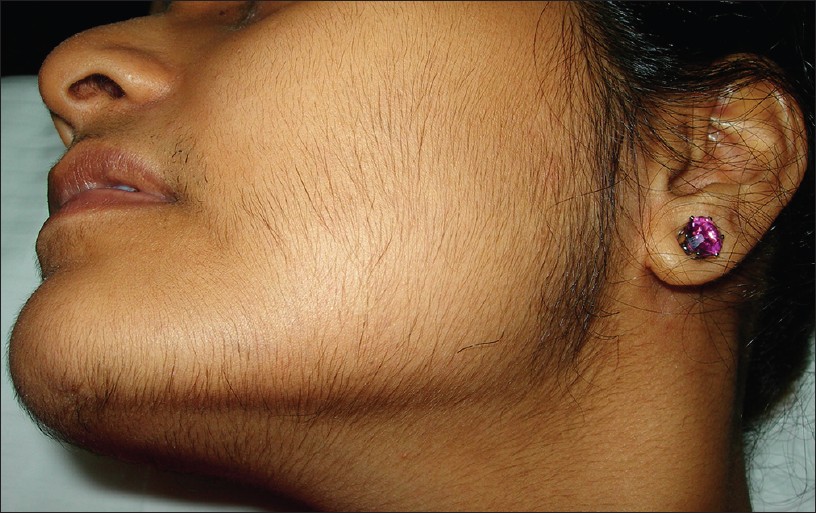 |
| Figure 4: Hirsutism in a patient with PCOS |
Alopecia
Not all cases of female pattern hair loss (FPHL) may be of androgenic origin. [18] Patterned hair loss in PCOS may be difficult to distinguish from those secondary to other hyperandrogenic states [Figure - 5]. Various clinical presentations include those described by Ludwig (diffuse), Hamilton (male pattern), and Olsen (frontal accentuation). [18] Women with early onset FPHL are much more likely to have an associated hyperandrogenism. Hormonal influences convert terminal to vellus hair, making the scalp appear bald.
 |
| Figure 5: Patterned hair loss with bi-temporal recession in a patient with PCOS |
Acanthosis nigricans
Typically thick dark velvety skin situated on the nape of the neck, axillae, groins and other frictional areas may often be the first clue of insulin resistance [Figure - 6]. The thickening occurs due to the stimulation of tyrosine kinase growth factor - signaling pathways in the epidermis. Insulin - like growth factor receptor 1 (IGF1R) is present in many tissues including the epidermis and ovary. High levels of insulin directly or indirectly stimulate the IGF1R resulting in the skin changes. Skin tags in the frictional areas like the neck, axillae, groins, infra mammary or even under a pendulous abdominal fold are common, especially in obese individuals.
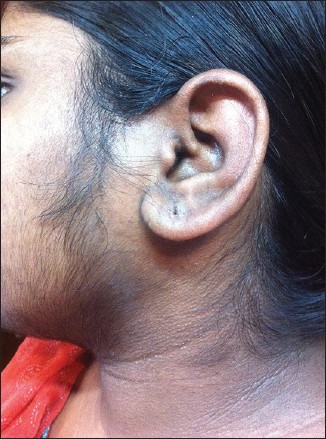 |
| Figure 6: Acanthosis nigricans and hirsutism in an obese girl with PCOS |
Irregular menses and infertility
PCOS is characterised by chronic menstrual irregularities or changes in menstrual pattern with reduced fertility. The anovulatory or oligoovulatory cycles result in continuous endometrial stimulation with estrogens, resulting in endometrial hyperplasia, thus increasing the risk of endometrial cancers. [19]
Metabolic complications
The incidence of type 2 Diabetes and gestational diabetes is markedly increased in both lean and obese women with PCOS. [4] Other morbidities include sleep apnoea, non-alcoholic liver disease, hypertension and dyslipidemia. The increased incidence of cardiovascular disease although probable, is still not supported in controlled studies.
Quality of Life Issues
Dokras et al. did a meta-analysis and systematic review of studies comparing anxiety symptoms in women with PCOS compared to normal, and concluded that all women with PCOS should be screened for anxiety symptoms. [20]
Diagnostic Criteria
Since 1990, various bodies have laid down criteria for the diagnosis of PCOS, based on oligo or anovulation, signs of hyperandrogenism, and ovarian sonography [Table - 2].

As mentioned in the Rotterdam diagnostic criteria, important causes of androgen excess must be ruled out before diagnosing PCOS and investigations should be directed towards detecting these conditions like late-onset congenital adrenal hyperplasia, hyperprolactinemia, pituitary tumors, ovarian tumors, etc., [Table - 3]. When a diagnosis of PCOS is made, it is almost one of exclusion.

Hormonal evaluation should be done in the pre-ovulatory phase. Since the levels of various hormones fluctuate considerably with a single menstrual cycle, it is best to carry out investigations in a fixed time within the cycle. The fourteenth day seems ideal, but since most patients have anovulatory or irregular cycles, the only fixed window for investigation would be immediately after the menstrual bleeding begins i.e. between the second to fourth day of the cycle. Normally, in this phase, FSH levels are 3 to 4 times higher than LH, but in patients with PCOS, this is reversed and a LH/FSH ratio greater than 2 is very suggestive of pituitary dysfunction.
A highly raised serum testosterone level (more than 3 times the upper normal range) is very suggestive of a neoplasm. If level of androstenedione is concomitantly elevated, it is almost diagnostic of an ovarian or adrenal malignancy, the former being much more frequent than the latter. Hence all patients with severely raised testosterone levels must undergo a trans-vaginal ultrasound to rule out any ovarian tumor. Adrenal tumors are more virilising and are associated with increased levels of DHEAS (>8 mmol/ml). The raised testosterone levels in such cases is secondary to increased peripheral conversion. A CT scan of the abdomen is the investigation of choice to rule out adrenal tumors.
Non-classical adrenal hyperplasia (NCAH) is a very close mimic to the PCOS and an 8:00 am serum 17-hydroxyprogesterone level should be done in suspected cases (normal is < 2 ug/ml). While a level of more than 8 ug/ml is diagnostic, it is the range in between (2-8 ug/ml) which requires further investigation. [21]
Prolactinoma may be an important cause for androgen excess. Although elevated plasma prolactin levels are highly suggestive of a prolactinoma, other causes of hyperprolactemia like hypothyroisim, Cushing′s disease, medications, etc., should be ruled out. Isolated increase in prolactin level is not uncommon in today′s stressful life, and its significance should be in context with the clinical presentation.
Insulin levels may be increased in a majority of patients with PCOS. The hyperinsulinemic-euglycemic clamp technique is gold standard for assessing insulin sensitivity, but is impractical in everyday practice. Rather, a ratio of fasting glucose to fasting insulin is a simple and effective alternative. A ratio of less than 7 is highly suggestive of a hyperinsulenemic state. In the authors′ experience, a fasting serum insulin level more than 15, and serum insulin level post 75gm glucose of more than 25 can also serve as a rough guide to a hyperinsulinemic status.
In 2003, Rotterdam criteria was the first to introduced the role of ultrasound examination of the ovaries as a diagnostic criteria. Although ovarian morphology is better depicted on trans-vaginal ultrasound examination, trans-abdominal ultrasound examination is preferred in adolescents and mandatory in unmarried women. Unlike blood investigations, the ultrasonography (USG) may be performed during any phase of the menstrual cycle but to avoid any confusion with a dominant follicle, it is best performed within few days of menstrual bleeding. The USG classically shows multiple subcentimeter cysts (>12, 2-9 mm) within the ovaries [Figure - 7]. The "string of pearls" appearance (peripheral arrangement of the follicles surrounding a central echogenic stroma), though characteristic, is not a requirement for diagnosis. The other ultrasonographic feature is that the volume of the ovaries should be each more than 10cc. These changes need not be seen in both ovaries to make a diagnosis of polycystic ovaries.
 |
| Figure 7: Ultrasound image of both ovaries showing multiple follicles arranged peripherally, giving a "string of pearls" appearance |
Investigations must be done to rule out underlying metabolic abnormality and include blood sugars and lipid profile. Physical evaluation must include BMI (wt/ht 2 ) calculation and whether patient has central obesity (waist: hip ratio), as these patients are at a higher risk of developing metabolic syndrome. [22]
Pcos Management
PCOS is a highly prevalent heterogeneous syndrome of clinical and/or biochemical androgen excess, ovulatory dysfunction and polycystic ovaries (PCO). Despite it being one of the most common reproductive health problems of women, its effective treatment remains a significant challenge to medical profession.
Treatment of women with PCOS tends to be symptom based. It is often difficult to treat all complaints at the same time. The most difficult one is the desire to treat both anovulatory infertility and hirsutism concurrently. Oral contraceptives are contra-indicated in infertility treatments because they block ovulation and anti-androgens in view of their potential teratogenic effects in a male fetus. Because of these conundrums in clinical care, treatment tends to fall into two categories, either the treatment of anovulatory infertility or the long term maintenance treatment for PCOS related symptoms (i.e., hirsutism, menstrual disorders, obesity, etc.).
Long Term Maintenance of Pcos
The term maintenance acknowledges the fact that there is no known cure for PCOS. Often the triad of hirsutism, oligomenorrhea, and obesity forms the key presenting symptoms. It may make sense to choose a primary metabolic parameter upon which to base initial treatment. Glucose intolerance is the strongest risk factor for diabetes and is also an independent risk factor for cardiovascular events in these women. Additional targeted therapies for hirsutism and/or oligomenorrhea could be added depending on response to the initial therapy. Contraception should be considered if the patient is trying to avoid pregnancy.
Therapies Aimed at Improving Insulin Sensitivity to Treat Anovulation and Androgen Excess
A logical approach to the management of PCOS includes using life style measures and medications that improve insulin sensitivity in target tissues, achieving reductions in insulin secretion, and stabilizing glucose tolerance.
Lifestyle modification
The gold standard for improving insulin sensitivity in obese PCOS women should be weight loss, diet, and exercise. It is recommended as the first line of treatment in obese women who present with infertility. Hypo-caloric diets result in appropriate weight loss in women with PCOS. Unfortunately, there have been few studies on the effect of exercise alone on symptoms in PCOS women, [23] although it is reasonable to assume that exercise would have the same beneficial effects in women with PCOS as it does in women with type 2 DM. However the exercise program must be tailored to the degree of obesity, and the patient′s baseline fitness.
Biguanide
Metformin may be most useful in the long term maintenance of PCOS. Metformin does lower serum androgens, and improves ovulatory and menstrual frequency. [24] One study estimated that menstrual frequency improved by roughly a third from baseline. Metformin is the drug of choice to treat glucose intolerance and elevated diabetes risk in women with PCOS. Its use throughout pregnancy has shown to have beneficial effects in reducing early pregnancy loss and have a favorable effect on plasma glucose levels and other metabolic aspects. [25] Metformin is also associated with weight loss in women with PCOS, although the results in other populations are inconsistent. Metformin is often used in conjunction with lifestyle therapy to treat PCOS. Gastrointestinal symptoms (diarrhea, nausea, vomiting, abdominal bloating, flatulence, and anorexia) are the most common adverse reactions and may be ameliorated by starting at a small dose and gradually increasing the dose or by using the sustained-release versions. The dose is usually 1500-2000 mg/day given in divided doses. Metformin carries a small risk of lactic acidosis, most commonly among women with poorly controlled diabetes and impaired renal function. Other reported side effects include vitamin B12 deficiency, [26] peripheral neuropathy, [27] and even hepatotoxicity. [28]
Thiazolidinediones
Pioglitazone and rosiglitazone are pharmacological ligands for the nuclear receptor peroxisome proliferator activated receptor γ (PPARγ). They improve the action of insulin in the liver, skeletal muscles, adipose tissue and have only modest effect on hepatic glucose output. Improving insulin sensitivity with these drugs is associated with a decrease in circulating androgen levels, improved ovulation rate, and improved glucose tolerance. [29],[30],[31],[32] However the concern about hepatotoxicity, cardiovascular risk, weight gain, and the pregnancy category C have limited the use of these drugs in women with PCOS.
Therapies Aimed at Treating Oligomenorrhea
If the patient does not wish to conceive, medical therapy is directed towards interruption of the effect of unopposed estrogen on the endometrium. Nonfluctuating levels of unopposed estradiol in the absence of progesterone cause irregular uterine bleeding, amenorrhea, infertility and increased risk of endometrial cancer.
Combination of oral contraceptives
Oral contraceptives have been the mainstay of long-term management of PCO. They offer benefit through a variety of mechanisms, including suppression of pituitary LH secretion, suppression of ovarian androgen secretion, and increased circulating SHBG levels. Individual OC preparations may have different doses and drug combinations and thus have varying risk-benefit ratios. Most oral contraceptives (OC) preperations contain estrogen (ethiny lestradiol 0.030 mg) in combination with anti-androgens. Anti-androgens include cyproterone acetate, drosperinone, levonorgestril, norgestimate and desogestril. The "best" oral contraceptive for women with PCOS is unknown.
Because women with PCOS may have multiple risk factors for serious adverse events on oral contraceptives, they must be screened carefully for risk factors for these events including smoking history, presence of obesity and hypertension, and history of clotting diathesis to mention some of the important factors. There is no convincing evidence that use of oral contraceptives contributes to the risk of diabetes in women with PCOS, although there are often adverse effects on insulin sensitivity that may be dose dependent. [33] A low dose oral contraceptive pill is therefore recommended. Oral contraceptives may also be associated with a significant elevation in circulating triglycerides as well as in high density lipoprotein (HDL) levels, though these do not appear to progress over time. There is no evidence to suggest that women with PCOS experience more cardiovascular events than the general population when they use oral contraceptives. If a woman is taking an oral contraceptive that contains drospirenone, a progestin with anti-mineralocorticoid properties, it may be necessary to reduce her dose of spironolactone if used concomitantly. Regular evaluation of potassium levels are necessary.
Progestin
Both depot and intermittent oral medroxyprogesterone acetate (10 mg for 10 days) have been shown to suppress pituitary gonadotropins and circulating androgens in women with PCOS. [34] No studies have addressed the long-term use of these compounds to treat hirsutism. There is also a paucity of data to address the varying risk benefit ratios of varying classes of progestins. Progestin-only oral contraceptives are an alternative for endometrial protection, but they are associated with a high incidence of breakthrough bleeding.
Therapies Aimed at Treating Hirsuitism
No oral contraceptive has been approved by the FDA for the treatment of hirsutism. A number of observational or non-randomized studies have noted improvement in hirsutism in women with PCOS who use oral contraceptives, but no studies of adequate power confirm their benefit in improving hirsutism in PCOS. [35]
Spironolactone
It is primarily used to treat hirsutism and appears effective, though the evidence is weak. It is a diuretic and aldosterone antagonist, also binds to the androgen receptor as an antagonist. It has other mechanisms of action, including inhibition of ovarian and adrenal steroidogenesis, competition for androgen receptors in hair follicles, and direct inhibition of 5 alpha reductase activity. The usual dose is 25-100 mg twice a day, and is titrated to balance efficacy while avoiding side effects such as orthostatic hypotension. A full clinical effect may take 6 months or more. About 20% of women using spironolactone will experience increased menstrual frequency. [36] Because it can cause and exacerbate hyperkalemia, spironolactone should be used cautiously in women with renal impairment. Rarely, exposure has resulted in ambiguous genitalia in male infants.
Cyproterone acetate
It is a progestogen with anti-androgen properties. It is frequently combined in an oral contraceptive tablets and is popular in the treatment of PCOS. A newer progestin from the same class, drospirenone has been marketed in the U.S. as especially effective for the treatment of female hyperandrogenism, although the data suggesting this is superior to other formulations is not based on head to head randomized trials. [37] When given as 100mg/day, it inhibits testosterone production resulting in upto 75% decrease in circulating testosterone levels.
Flutamide
It is an androgen-receptor antagonist, and is another non steroidal anti-androgen that has been shown to be effective against hirsutism in smaller trials The most common side effect is dry skin, but its use has been associated with hepatitis in rare cases. The common dosage is 250 mg/day. The risk of teratogenicity with this compound is significant, and contraception should be used. Flutamide has also been combined with lifestyle and metformin therapy for treatment of PCOS and may have additive effects. [38]
Finasteride
It is a specific inhibitor of type II 5α reductase enzyme found in the hair follicles on the top of the scalp and in the sebaceous gland ducts. Its use is restricted to women in the post-menopausal group or women with documented hyperandrogenic state in the dosage of 5 mg/day. Finasteride is better tolerated than other anti-androgens, with minimal hepatic and renal toxicity; however, it has well-documented risk for teratogenicity and feminising in a male fetuses, and adequate contraception should be used. Overall, randomized trials have found that spironolactone, flutamide and finasteride to have similar efficacy in improving hirsutism.
Ornithine decarboxylase inhibitors
These have been developed for the treatment of female hirsutism. Ornithine decarboxylase is necessary for the production of polyamines, and inhibition of this enzyme limits cell division and function in the pilosebaceous unit. Recently a potent inhibitor of this enzyme, eflornithine, has been found to be effective as a facial cream for the treatment of unwanted facial hair. [39] It is available as a 13.9% cream of eflornithine hydrochloride, and is applied to affected areas twice daily. In clinical trials, 32% of patients had marked improvement after 24 weeks compared to 8% of placebo treated women, and the benefit was first noted at eight weeks. It is pregnancy category C drug. It appears to be well tolerated, with only about 2% of patients developing skin irritation or other adverse reactions. Relapse is common after stopping.
Mechanical and cosmetic means of hair reduction and destruction
Mechanical hair removal techniques like shaving, plucking, waxing, depilatory creams, electrolysis, and laser hair reduction (LHR) offer good cosmetic relief and often are the front line of treatment used by women. A word of caution regarding facial waxing as it can precipitate folliculitis. Various lasers are used for hair reduction and include the Diode (800nm), Alexandrite (755 nm), Long pulsed Nd-YAG (neodymium-doped yttrium aluminium garnet; Nd: Y3Al5O12) (1064nm) and IPL devices. There is no "gold standard" for LHR but the long pulsed Nd-YAG is the safest in pigmented skins. It must be emphasized that LHR would be effective only after anti-androgen therapy has been initiated at least 2 to 3 months prior. It results in reduction in density and thickness of hair but if at any point in time, there is uncontrolled androgen excess, there will be a relapse in hirsutism.
Therapies Aimed at Treating Anovulatory Infertility
The recommended first-line treatment for ovulation induction remains the anti-estrogen clomiphene citrate (CC). Recommended second-line intervention is either exogenous gonadotropins or laparoscopic ovarian surgery. [40] There appears to be some benefit of addition of metformin to clomiphene, especially in obese subjects (modified first line treatment).
Clomiphene citrate
It is a triphenylethylene derived nonsteroidal agent that is theorized to function at the level of the hypothalamus as an anti-estrogen to improve gonadotropin secretion. An important concern is the relatively high rate of multiple pregnancies (7.8%) after conception, majority being twins. [41]
Gonadotropins
These are frequently used to induce ovulation in women with PCOS for whom clomiphene treatment has failed. Low-dose therapy with gonadotropins offers a higher rate of ovulation, monofollicular development, with a significantly lower risk of ovarian hyperstimulation syndrome. [42] Pregnancy rates are comparable with other regimens, but there have been no adequately powered trials to answer this question.
Ovarian surgery
This is primarily recommended as second line infertility therapy. Multiple pregnancy rates are reduced in those women who conceive following laparoscopic drilling. In some cases, the fertility benefits of ovarian drilling may be temporary and adjuvant therapy after drilling with clomiphene may be necessary. [43] Ovarian drilling does not appear to improve metabolic abnormalities in women with PCOS. [44]
Aromatase inhibitors
Aromatase inhibitors such as letrozole and anastrazole have been proposed as both first and secondary treatment for ovulation induction (in women with PCOS and also for unexplained infertility). [45] Results in women with PCOS appear comparable to clomiphene from small trials. Proposed benefits include oral administration, a shorter half life than clomiphene, more favorable effects on the endometrium, potentially higher implantation rates, and lower multiple pregnancy rates due to monofollicular ovulation. Their use is still experimental at this point.
Thiazolidinediones
Smaller trials have shown some benefit to this class of drugs for the treatment of infertility usually in conjunction with clomiphene. [46],[47] However the concern about hepatotoxicity, cardiovascular risk, weight gain, and the pregnancy Category C have limited the use of these drugs in women with PCOS.
Research Ongoing
Statins
Another area where there is emerging support in the literature for a cardiovascular and endocrine benefit in women with PCOS is the use of statins. They have been shown to improve hyperandrogenemia, lipid levels, and reduce inflammation. [48] However, their long term effects in preventing cardiovascular disease in young women with PCOS is unknown. There are concerns about teratogenecity with the use of this drug in reproductive age women, as it is FDA pregnancy category X. The use of these drugs is still experimental in women with PCOS.
Approach to a Patient with PCOS
Suspect PCOS in women (obese/lean) with/without a history of irregular periods with one or more of the following complaints, namely, acne, hirsutism, alopecia, acanthosis nigricans and metabolic syndrome. Investigations both, hematological and imaging are geared to rule out other hyperandrogenic states as there is no single investigation to diagnose PCOS. Patients must be counseled about the long duration of treatment which includes life-style modifications along with the systemic treatment.
Success in effective management of a woman with PCOS is through a synchronized effort between the dermatologist, endocrinologist, gynecologist, nutritionist and physical trainer.
| 1. |
Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935;29:181-91.
[Google Scholar]
|
| 2. |
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745-9.
[Google Scholar]
|
| 3. |
Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol 2011;24:223-7.
[Google Scholar]
|
| 4. |
Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 1999;84:165-9.
[Google Scholar]
|
| 5. |
Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM. Australian ovarian cancer study group and australian national endometrial cancer study group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: An Australian case-control study. Cancer Causes Control 2010;21:2303-8.
[Google Scholar]
|
| 6. |
Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril 1994;62:20-7.
[Google Scholar]
|
| 7. |
Dunaif A. Insulin resistance and the polycystic ovary syndrome mechanism and implications for pathogenesis. Endocr Rev 1997;18:774-800.
[Google Scholar]
|
| 8. |
Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:1-17.
[Google Scholar]
|
| 9. |
Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity in polycystic ovary syndrome. Diabetes 1989;38:1165-74.
[Google Scholar]
|
| 10. |
Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 1988;123:733-9.
[Google Scholar]
|
| 11. |
Shi D, Vine DF. Animal models of polycystic ovary syndrome: A focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril 2012;98:185-93.
[Google Scholar]
|
| 12. |
Xita N, Tsatsoulis A. Review: Fetal programming of polycystic ovary syndrome by androgen excess: Evidence from experimental, clinical and genetic association studies. J Clin Endocrinol Metab 2006;91:1660-6.
[Google Scholar]
|
| 13. |
Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2002;87:2134.
[Google Scholar]
|
| 14. |
Leibel NI, Baumann EA, Kocherginsky M, Rosenfield RL. Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J Clin Endocrinol Metab 2006;91:1275-83.
[Google Scholar]
|
| 15. |
Archer JS, Chang RJ. Hirsutism and acne in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2004;18:737-54.
[Google Scholar]
|
| 16. |
Azziz R. The evaluation and management of hirsutism. Obstet Gynecol 2003;101:995-1007.
[Google Scholar]
|
| 17. |
Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab 2000;85:3161-8.
[Google Scholar]
|
| 18. |
Olsen EA. Female pattern hair loss. J Am Acad Dermatol 2001;45:70-80.
[Google Scholar]
|
| 19. |
Park JC, Lim SY, Jang TK, Bae JG, Kim JI, Rhee JH. Endometrial histology and predictable clinical factors for endometrial disease in women with polycystic ovary syndrome. Clin Exp Reprod Med 2011;38:42-6.
[Google Scholar]
|
| 20. |
Dokras A, Clifton S, Futterweit W, Wild R. Quality of Life Issues and PCOS. Fertil Steril 2012;97:225-30.
[Google Scholar]
|
| 21. |
Escobar-Morreale HF, Sanchon R, San Millan JL. A prospective study of the prevalence of nonclassical congenital adrenal hyperplasia among women presenting with hyperandrogenic symptoms and signs. J Clin Endocrinol Metab 2008;93:527-33.
[Google Scholar]
|
| 22. |
Mandrelle K, Kamath MS, Bondu DJ, Chandy A, Aleyamma T, George K. Prevalence of metabolic syndrome in women with polycystic ovarysyndrome attending an infertility clinic in a tertiary care hospital in south India. J Hum Reprod Sci 2012;5:26-31.
[Google Scholar]
|
| 23. |
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.
[Google Scholar]
|
| 24. |
Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 2003;327:951-3.
[Google Scholar]
|
| 25. |
Kumar P, Khan K. Effects of metformin use in pregnant patients with polycystic ovary syndrome. J Hum Reprod Sci 2012;5:166-9.
[Google Scholar]
|
| 26. |
Kumthekar AA, Gidwani HV, Kumthekar AB. Metformin associated B12 deficiency. J Assoc Physicians India 2012;60:58-60.
[Google Scholar]
|
| 27. |
Bell DS. Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy. South Med J 2010;103:265-7.
[Google Scholar]
|
| 28. |
Miralles-Linares F, Puerta-Fernandez S, Bernal-Lopez MR, Tinahones FJ, Andrade RJ, Gomez-Huelgas R. Metformin-induced hepatotoxicity. Diabetes Care 2012;35:e21.
[Google Scholar]
|
| 29. |
Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: A multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2001;86:1626-32.
[Google Scholar]
|
| 30. |
Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril 2004;82:893-902.
[Google Scholar]
|
| 31. |
Vessey MP, Painter R. Endometrial and ovarian cancer and oral contraceptives-findings in a large cohort study. Br J Cancer 1995;71:1340-2.
[Google Scholar]
|
| 32. |
Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives. A practitioner's guide to meta-analysis. Hum Reprod 1997;2:1851-63.
[Google Scholar]
|
| 33. |
Costello MF, Shrestha B, Eden J, Johnson NP, Sjoblom P. Metformin versus oral contraceptive pill in polycystic ovary syndrome: A Cochrane review. Hum Reprod 2007;22:1200-9.
[Google Scholar]
|
| 34. |
Anttila L, Koskinen P, Erkkola R, Irjala K, Ruutiainen K. Serum testosterone, androstenedione and luteinizing hormone levels after short-term medroxyprogesterone acetate treatment in women with polycystic ovarian disease. Acta Obstet Gynecol Scand 1994;73:634-6.
[Google Scholar]
|
| 35. |
Swiglo BA, Cosma M, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Antiandrogens for the treatment of hirsutism: A systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008;93:1153-60.
[Google Scholar]
|
| 36. |
Helfer EL, Miller JL, Rose LI. Side-effects of spironolactone therapy in the hirsute woman. J Clin Endocrinol Metab 1988;66:208-11.
[Google Scholar]
|
| 37. |
Palep-Singh M, Mook K, Barth J, Balen A. An observational study of Yasmin in the management of women with polycystic ovary syndrome. J Fam Plann Reprod Health Care 2004;30:163-5.
[Google Scholar]
|
| 38. |
Gambineri A, Patton L, Vaccina A, Cacciari M, Morselli-Labate AM, Cavazza C, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: A randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 2006;91:3970-80.
[Google Scholar]
|
| 39. |
Wolf JE Jr, Shander D, Huber F, Jackson J, Lin CS, Mathes BM, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol 2007;46:94-8.
[Google Scholar]
|
| 40. |
Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 2008;89:505-22.
[Google Scholar]
|
| 41. |
Asch RH, Greenblatt RB. Update on the safety and efficacy of clomiphene citrate as a therapeutic agent. J Reprod Med 1976;17:175-80.
[Google Scholar]
|
| 42. |
Christin-Maitre S, Hugues JN. A comparative randomized multicentric study comparing the step-up versus step-down protocol in polycystic ovary syndrome. Hum Reprod 2003;18:1626-31.
[Google Scholar]
|
| 43. |
Farquhar C, Brown J, Marjoribanks J. Laparoscopic �drilling� by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev 2012;6:CD001122.
[Google Scholar]
|
| 44. |
Lemieux S, Lewis GF, Ben-Chetrit A, Steiner G, Greenblatt EM. Correction of hyperandrogenemia by laparoscopic ovarian cautery in women with polycystic ovarian syndrome is not accompanied by improved insulin sensitivity or lipid-lipoprotein levels. J Clin Endocrinol Metab 1999;84:4278-82.
[Google Scholar]
|
| 45. |
Gysler M, March CM, Mishell DR Jr, Bailey EJ. A decade�s experience with an individualized clomiphene treatment regimen including its effect on the postcoital test. Fertil Steril 1982;37:161-7.
[Google Scholar]
|
| 46. |
Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D, Ke RW. Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertil Steril 2003;79:562-6.
[Google Scholar]
|
| 47. |
Rouzi AA, Ardawi MS. A randomized controlled trial of the efficacy of rosiglitazone and clomiphene citrate versus metformin and clomiphene citrate in women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil Steril 2006;85:428-35.
[Google Scholar]
|
| 48. |
Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled study. J Clin Endocrinol Metab 2009;94:103-8.
[Google Scholar]
|
Fulltext Views
23,716
PDF downloads
4,163





