Translate this page into:
Ponatinib-induced atypical pityriasis rubra pilaris-like rash
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Rahul Mahajan
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh
India
| How to cite this article: Kamat D, Chatterjee D, Mahajan R. Ponatinib-induced atypical pityriasis rubra pilaris-like rash. Indian J Dermatol Venereol Leprol 2020;86:688-690 |
Sir,
The introduction of targeted chemotherapeutic agents like tyrosine kinase inhibitors elicit more selective anti-cancer effects and have lesser systemic toxicities when compared to traditional chemotherapeutic agents. Ponatinib, a third generation tyrosine kinase inhibitor, was approved by Food and Drug Administration (FDA) in 2012 for treatment refractory chronic myelogenous leukemia (CML) and Philadelphia chromosome positive (Ph+) acute lymphocytic leukemia.[1],[2] We present a patient with refractory CML who presented with an atypical skin rash after institution of ponatinib.
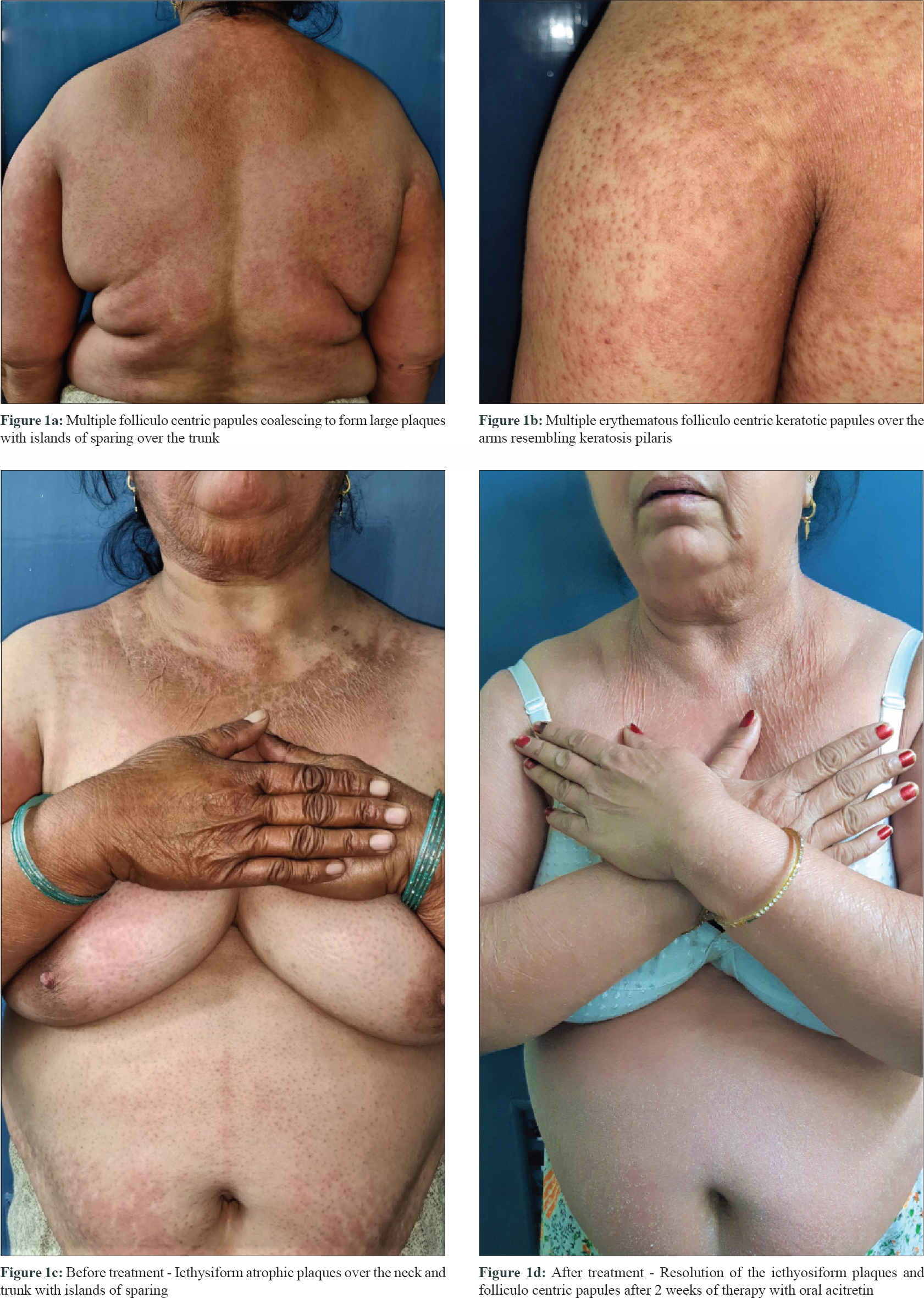
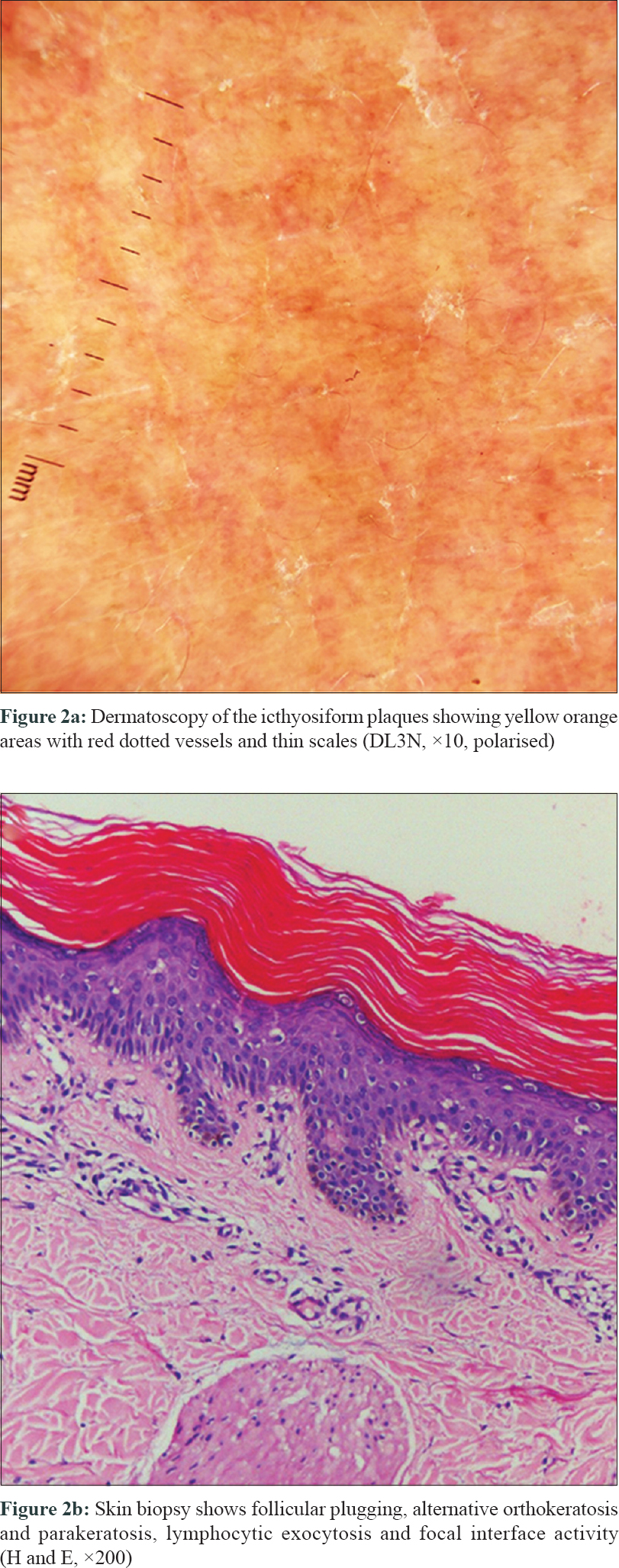
A 52-year-old woman presented to our out-patient department with complaints of an itchy skin eruption of one month duration. She had been diagnosed with BCR/ABL positive chronic myeloid leukemia 2 years back, but had failed to respond to the first line treatment. Oral Ponatinib 45mg once daily was thus initiated. Three weeks after starting the drug, she developed redness and itchy eruption initially over face and forearms which gradually extended to involve the trunk. Examination revealed multiple reddish folliculo centric papules distributed symmetrically over the trunk and upper arms resembling keratosis pilaris [Figure - 1]a and [Figure - 1]b. Irregular icthyosiform atrophic plaques were seen over the upper chest and neck with islands of sparing [Figure - 1]c. Dorsum of hands, forearms and legs had a diffuse atrophic wrinkled appearance. However, palmoplantar keratoderma was conspicuously absent. Dermatoscopy highlighted the yellow orange areas with red dotted vessels and thin scales [Figure - 2]a. Histopathology showed follicular plugging, alternative orthokeratosis and parakeratosis, lymphocytic exocytosis and focal interface activity [Figure - 2]b. A diagnosis of atypical pityriasis rubra pilaris (PRP)-like rash was thus made and topical tazarotene 0.05% cream was advised to be applied once daily over the forearms. There was no photosensitivity following topical application of tazarotene. Due to progressive lesions, oral acitretin 25 mg once daily was added without discontinuing ponatinib. Tazarotene was stopped due to extensive involvement and emollients were prescribed instead. After 2 weeks of treatment with acitretin, there was significant response with near complete resolution of the folliculocentric and icthyotic lesions [Figure - 1]d Acitretin was further continued along with ponatinib. Acitretin was continued for 10 weeks following which patient was lost to follow up. The dose was maintained at 25 mg once daily and was not hiked as there was satisfactory improvement with this.
 |
 |
The best evidence regarding frequency and management of skin toxicity due to ponatinib comes from few large randomized controlled trials such as the PACE trial in which dermatologic specific adverse effects were reported to be rash (47%) and dry skin (42%).[3] The mechanism of these icthyosiform eruptions has been poorly elucidated. Another common cutaneous eruption reported with tyrosine kinase inhibitors is keratosis pilaris-like lesions which are also seen with Rapidly Accelerated Fibrosarcoma Gene (RAF) inhibitors. In addition to being a tyrosine kinase inhibitor, ponatinib also causes 'off target' inhibition of various other signaling pathways like fibroblast growth factor, platelet derived growth factor and sarcoma (SRC) family proteins amongst others. This might account for the similarity of cutaneous eruptions seen with other multikinase inhibitors.[4]
The index case had a type 2 PRP-like rash similar to the previously reported cases.[4],[5] Most of these cases had favorable response to treatment without discontinuing Ponatinib, although one needed Ponatinib dose reduction. Treatment options include topical steroids and retinoids. For more generalized cases oral corticosteroids, oral retinoids, phototherapy or combination of these have been successfully tried as in our patient.[4],[5]
Systemic retinoids have also been used as a chemo-preventive agent in hematological malignancies, especially acute promyelocytic leukemia. All-trans retinoic acid induces differentiation of cancer stem cells and modulates the cell cycle.In vitro studies have also shown a suppressive effect of All trans retinoic acid on Ph+ cells in CML.[6] In conclusion, dermatological side effects especially icthyosiform eruptions are fairly common with ponatinib. Oral retinoids are effective for generalized PRP-like eruptions and most cases do not require discontinuation of ponatinib.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Alloo A, Sheu J, Butrynski JE, de Angelo DJ, George S, Murphy GF, et al. Ponatinib-induced pityriasiform, folliculocentric and ichthyosiform cutaneous toxicities. Br J Dermatol 2015;173:574-7.
[Google Scholar]
|
| 2. |
Derlino F, Barruscotti S, Zappasodi P, Brazzelli V, Vassallo C. Ponatinib-induced widespread ichthyosiform eruption. J Eur Acad Dermatol Venereol 2017;31:e519-21.
[Google Scholar]
|
| 3. |
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: Final 5-year results of the phase 2 PACE trial. Blood 2018;132:393-404.
[Google Scholar]
|
| 4. |
Eber AE, Rosen A, Oberlin KE, Giubellino A, Romanelli P. Ichthyosiform pityriasis rubra pilaris-like Eruption Secondary to Ponatinib Therapy: Case report and literature review. Drug Saf Case Rep 2017;4:19.
[Google Scholar]
|
| 5. |
Jack A, Mauro MJ, Ehst BD. Pityriasis rubra pilaris-like eruption associated with the multikinase inhibitor ponatinib. J Am Acad Dermatol 2013;69:e249-50.
[Google Scholar]
|
| 6. |
Bunaciu RP, Yen A. Retinoid chemoprevention: Who can benefit? Curr Pharmacol Rep 2015;1:391-400.
[Google Scholar]
|
Fulltext Views
4,259
PDF downloads
1,555





