Translate this page into:
Post-kala-azar dermal leishmaniasis: A histopathological study
2 Departments of Pathology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Sanjay K Rathi
Dr. Rathi's Skin Clinic, 143, Hill Cart Road, Siliguri - 734401, W. Bengal
India
| How to cite this article: Rathi SK, Pandhi R K, Chopra P, Khanna N. Post-kala-azar dermal leishmaniasis: A histopathological study. Indian J Dermatol Venereol Leprol 2005;71:250-253 |
Abstract
BACKGROUND : Post-kala-azar dermal leishmaniasis follows an attack of visceral leishmaniasis and is caused by the same organism, i.e. Leishmania donovani. METHODS: In the present study, biopsy specimens from hypopigmented macules, nodules or plaques of 25 patients clinically diagnosed as PKDL were evaluated for epidermal and dermal changes and for the presence or absence of Leishmania donovani bodies (LDBs). RESULTS : The hypopigmented macules showed a patchy perivascular and periappendageal infiltrate with no demonstrable LDBs in any of the biopsies. In the nodular and plaque lesions, the infiltrate was diffuse, beneath an atrophic epidermis (74%) and follicular plugging (95.6%) was seen in most biopsies. The infiltrate consisted of lymphocytes, histiocytes and plasma cells in decreasing order of presence. LDBs could be demonstrated in only 10 (43.5%) biopsy specimens from nodular and plaque lesions and were never numerous. CONCLUSIONS: Histopathological features of PKDL are elucidated and discussed.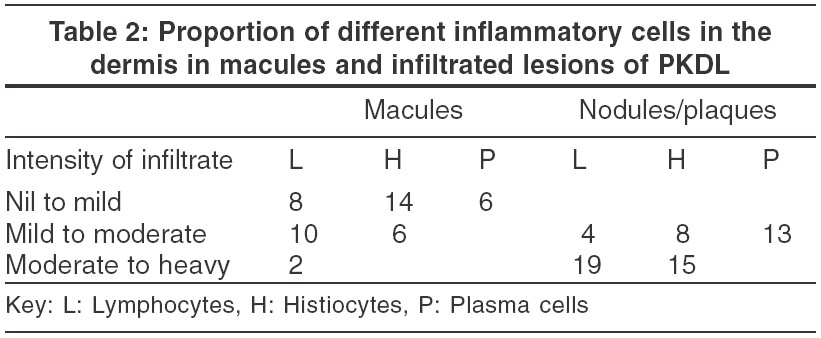


 |
 |
 |
 |
 |
 |
Introduction
Post kala-azar dermal leishmaniasis (PKDL) usually develops 6 months-5 years following an attack of untreated or incompletely treated visceral leishmaniasis (VL).[1],[2] Both PKDL and VL are caused by the same organism, a protozoa, Leishmania donovani .[3] Three types of clinical manifestations have been described in PKDL: hypopigmented macules, nodules and plaques and erythema of face. Though not an infrequent disease in endemic areas, there is paucity of reports on the histopathological findings in PKDL. The present study focuses on the histopathology of the hypopigmented macules, nodules and plaques of patients with PKDL.
Materials and Methods
Twenty-five patients with clinical lesions suggestive of PKDL (macules, nodules and plaques) who attended the Dermatology OPD of the All India Institute of Medical Sciences, New Delhi over a period of two years were recruited in the present study. Tissue smears were made from both types of skin lesions and stained with Giemsa stain to demonstrate Leishmania Donovani bodies (LDBs). Slit-skin smears for acid-fast bacilli were also made, to rule out leprosy.
The biopsy specimens were taken from a macule, nodule or plaque depending on the clinical presentation of the patient. Sections were stained with hematoxylin and eosin (H&E) and special stains, Fite (for demonstration of AFB), and Giemsa (for demonstration of LDBs) and examined for:
· Epidermal changes, if any.
· Dermal changes, including intensity, pattern and type of inflammatory cell infiltrate. The intensity of the infiltrate was graded subjectively from nil to mild, mild to moderate and moderate to heavy. Presence or absence of LDBs.
Results
The 25 patients included 22 (88%) males and 3 (12%) females. Their age ranged from 6 to 55 years. Twenty (80%) patients gave a history of preceding Kala-azar.
Morphological profile Two main morphological types of lesions were seen: hypopigmented macules, nodules and plaques. Two (8%) patients had only macules, 5 (20%) only nodules and plaques, while 18 (72%) had all types of lesions.
Tissue smear LDBs were demonstrated either within or outside the macrophages by Giemsa stained smears from infiltrated plaques and nodules in 11 (47.8%) of 23 patients, but in none of the macular lesions in the 20 patients who had such lesions. Slit-skin smears for acid-fast bacilli were negative in all cases.
Histopathological features Forty-three biopsy specimens were processed, 20 from macules and 23 from nodules.
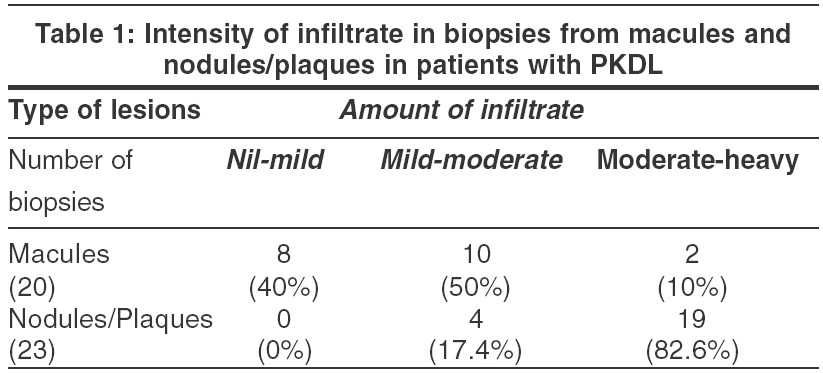
Epidermal changes Five (20%) of the 20 biopsies taken from the macules and 17 (74%) of the 23 biopsies from nodules showed epidermal atrophy [Figure - 1]. Twenty-two (95.6%) biopsies from infiltrated nodules and plaques [Figure - 1] and 9 (45%) from macules showed follicular plugging, especially in lesions from the face and axillary folds.
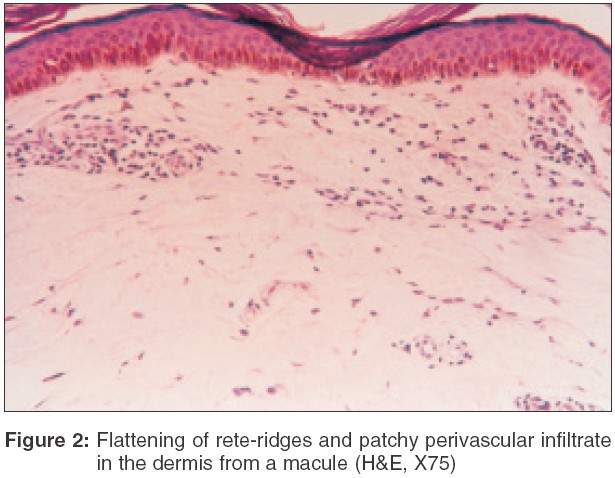
Dermal changes Nineteen (83%) of the 23 biopsies from nodules had a moderate to heavy diffuse dermal infiltrate [Table - 1] while 4 (17%) biopsy specimens showed a mild-moderate patchy infiltrate. In 11 (55%) biopsies from macular lesions, the infiltrate was patchy. When the infiltrate was diffuse, it was located mainly in the upper and mid-dermis while the patchy infiltrate was perivascular and periappendageal [Figure - 2]. [Table - 1] shows the amount of infiltrate in macular lesions, nodules and plaques. The infiltrate consisted predominantly of lymphohistiocytes admixed with plasma cells. When the infiltrate was moderate to heavy, plasma cells were inconspicuous; on the other hand, when the infiltrate was mild to moderate, plasma cells were conspicuous both in the macules and infiltrated lesions [Table - 2].
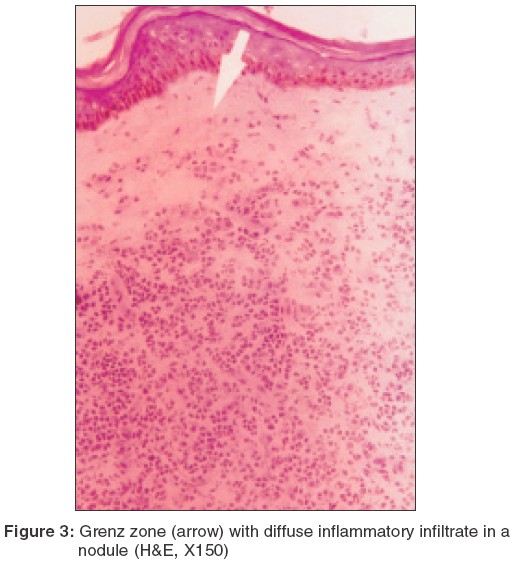
A subepidermal clear zone, was seen in 11 (48%) biopsy specimens from nodules [Figure - 3] and in 3 (15%) specimens from macules. Pigment incontinence was seen in 10 (43%) biopsy specimens taken from nodules and in 5 (25%) from macules.
Special stains LDBs either within the histiocytes or extracellularly could be demonstrated in 10 (40%) biopsy specimens from nodules/plaques. LDBs were never numerous and were mainly seen in the upper and mid-dermis and only after a thorough search. No LDBs could be demonstrated in macular lesions. Giemsa staining did not have any advantages over H/E staining in demonstrating LDBs. The Fite stain for M. leprae was negative in all patients.
Discussion
PKDL is the sequel of untreated or partially treated KA which characteristically manifests as macules, nodules, plaques and facial erythema. A minimal mixed chronic inflammatory infiltrate consisting of plasma cells, lymphocytes and histiocytes in the perivascular areas of the upper dermis has been described in macules of PKDL.[1],[2],[3],[4] In our study, the infiltrate consisted mainly of lymphocytes, histiocytes and occasional plasma cells, corroborating the findings of Singh[5] and Ramesh et al.[6] Pigment dilution in basal layers has also been reported in macules.[7] We found atrophy (25%) and follicular plugging (45%) as additional epidermal changes in the macular lesions.
As regards the infiltrated nodules and plaques, the findings of earlier studies have varied from a macrophage granuloma with heavy parasitization to an epithelioid cell granuloma with minimal presence of LDBs.[2],[3],[5],[7],[8],[9],[10],[11] The granuloma generally comprises lymphocytes, histiocytes and plasma cells in varying proportions. Singh et al described a dense inflammatory infiltrate of mononuclear cells, histiocytes and plasma cells with sparse LDBs in the upper dermis, and a narrow sub-epidermal Grenz zone.[10] The epidermis was atrophic and follicular plugging was a prominent feature. This suggests a spectrum of histopathological findings in PKDL.
In our study, epidermal atrophy (74%) and follicular plugging (95.6%) were conspicuous in a vast majority of nodular lesions. In the dermis, a Grenz zone was seen in almost (48%) half of the specimens along with diffuse infiltrate (83%) which was placed in the upper and mid-dermis. The infiltrate consisted of lymphocytes, admixed with histiocytes and plasma cells, but there was no tendency to granuloma formation. Mukherjee et al[11] and Ramesh et al[6] reported that plasma cells were the most predominant cells in the biopsy specimens of nodules, but our study did not corroborate this observation. Interestingly, we observed that plasma cells were more conspicuous when the infiltrate was sparse. Pigment incontinence was seen in 43% of the nodular lesions.
In earlier studies, the number of LDBs has not been large, and, more importantly, their number was not compatible with the extent and type of skin lesions. Our findings support this, as in 52% of patients, LDBs could not be demonstrated, even from the nodular lesions. This paucity of LDBs may be explained by the leishmanicidal effect of rifampicin, which is often prescribed in these patients due to concomitant tuberculosis or even for their PKDL. Another reason could be the good host-parasite relationship that allows the organism to lie dormant until conditions favor its multiplication.[6]
| 1. |
Das Gupta BM. A note on the parasite of dermal leishmanoid. Indian Med Gaz 1927;62:11-2.
[Google Scholar]
|
| 2. |
Brahmachari UN. A new form of cutaneous leishmaniasis - dermal leishmanoid. Indian Med Gaz 1923;57:1125-7.
[Google Scholar]
|
| 3. |
Acton HW, Napier LE. Post-kala-azar dermal leishmaniasis. Indian Med Gaz 1929;64:147-8.
[Google Scholar]
|
| 4. |
Sen Gupta PC, Bhattacharjee B. Histopathology of post kala-azar dermal leishmaniasis. J Trop Med Hyg 1953;56:110-6.
[Google Scholar]
|
| 5. |
Singh RP. Observations on dermal leishmanoid in Bihar. Ind J Dermatol 1968;13:59-63.
[Google Scholar]
|
| 6. |
Ramesh V, Misra RS, Saxena U, Mukherjee A. Post kala-azar dermal leishmaniasis: a clinical and therapeutic study. Int J Dermatol 1993;32:272-5.
[Google Scholar]
|
| 7. |
Yawalkar SJ, Mardhekar BV, Mahabir BS. Post kala-azar dermal leishmaniasis. J Trop Med Hyg 1966;69:140-2.
[Google Scholar]
|
| 8. |
Ramesh V, Singh N. A clinical and histopathological study of macular type of post kala-azar dermal leishmaniasis. Trop Doct 1999;29:205-7.
[Google Scholar]
|
| 9. |
Girgla HS, Maarsden RS, Singh GM, Ryan TJ. Post kala-azar dermal leishmaniais. Brit J Dermatol 1997;97:307-9.
[Google Scholar]
|
| 10. |
Singh N, Ramesh V, Arora VK, Bhatia A, Kubba A, Ramam M. Nodular post kala-azar dermal leishmaniasis: a distinct histopathological entity. J Cutan Pathol 1998;25:95-9.
[Google Scholar]
|
| 11. |
Mukherjee A, Ramesh V, Misra RS. Post-kala-azar dermal leishmaniasis: A light and electron microscopic study of 18 cases. J Cutan Pathol 1993;20:320-5.
[Google Scholar]
|
Fulltext Views
4,059
PDF downloads
2,388





