Translate this page into:
Postsurgical pyoderma gangrenosum successfully treated with cyclosporine
2 Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
3 Department of General Surgery, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
Corresponding Author:
Laxmisha Chandrashekar
Associate Professor, Department of Dermatology, Venereology and Leprology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry - 605 006
India
laxmishac@gmail.com
| How to cite this article: Behera B, Chandrashekar L, Thappa DM, Srinivas BH, Pasayat PP, Laroiya I. Postsurgical pyoderma gangrenosum successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol 2017;83:412 |
Sir,
A 67-year-old man presented to the surgery department with anorexia, progressive constipation and weight loss for one month. The contrast-enhanced computed tomography of the abdomen revealed inflammatory swelling of the proximal jejunum extending into the mesentery. The mass was removed surgically, followed by a gastrojejunostomy. On the 5th post-operative day, he was referred to the dermatology department for low-grade fever and gaping of the surgical wound, which was associated with rapidly progressive erythema and necrosis. The patient also had a history of multiple ulcers at sites of minor trauma, which used to heal with atrophic scars. He had history of diabetes for last 8 years and was on tablet metformin 500 mg three times per day. There was no history suggestive of inflammatory bowel disease, connective tissue disorders, recurrent infection, bleeding or hepatic disorders. Cutaneous examination revealed extensive necrosis of the midline abdominal surgical incision site with formation of an ulcer that was surrounded by erythematous and edematous unhealthy skin. The floor of the ulcer was covered with purulent slough [Figure - 1]. He was pale, febrile (100° F) and had tachycardia (110 beats/min). Biopsies and tissue cultures were taken from the wound edge for suspected postoperative pyoderma gangrenosum. Histopathological examination revealed necrosis of the epidermis with a dense infiltrate of neutrophils. The dermis showed an extensive neutrophilic infiltrate forming microabscesses, perivascular lymphocytic infiltrate and occasional fibrinoid necrosis of the vessel wall [Figure - 2]. Cultures of tissue specimens for bacteria, fungus and mycobacteria were negative. Histopathology of the abdominal mass was consistent with an inflammatory growth that developed due to a jejunal stricture after healing of a perforation. The hemogram revealed microcytic hypochromic anemia and neutrophilic leukocytosis. Other investigations were within normal limits. The patient was started on oral cyclosporine 100 mg twice daily (3 mg/kg/day). The wound dressing was done using calcium alginate and debridement was avoided. After two months of follow-up, the lesion healed almost completely [Figure - 3]. The patient was given a maintenance dose of cyclosporine 50 mg dailyand was advised to continue for 3 months and follow-up regularly.
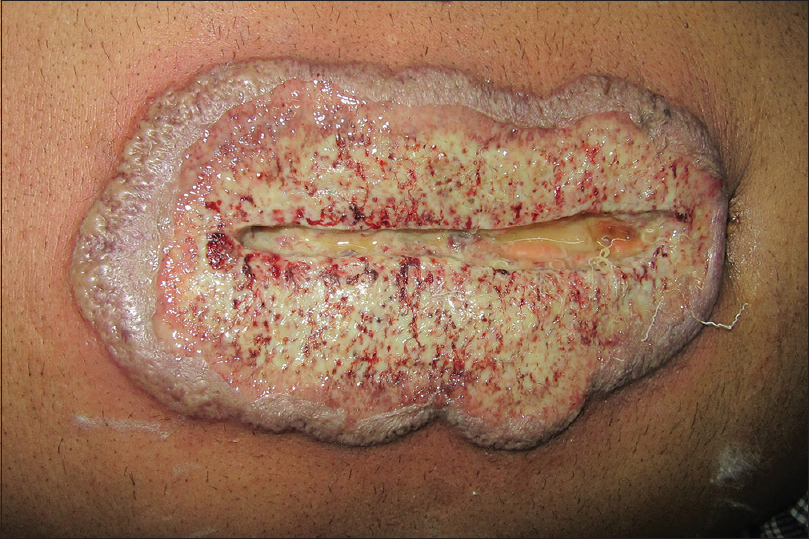 |
| Figure 1: Massive necrosis of the surgical wound site with prominent erythema and edema suggesting the active nature of postsurgical pyoderma gangrenosum |
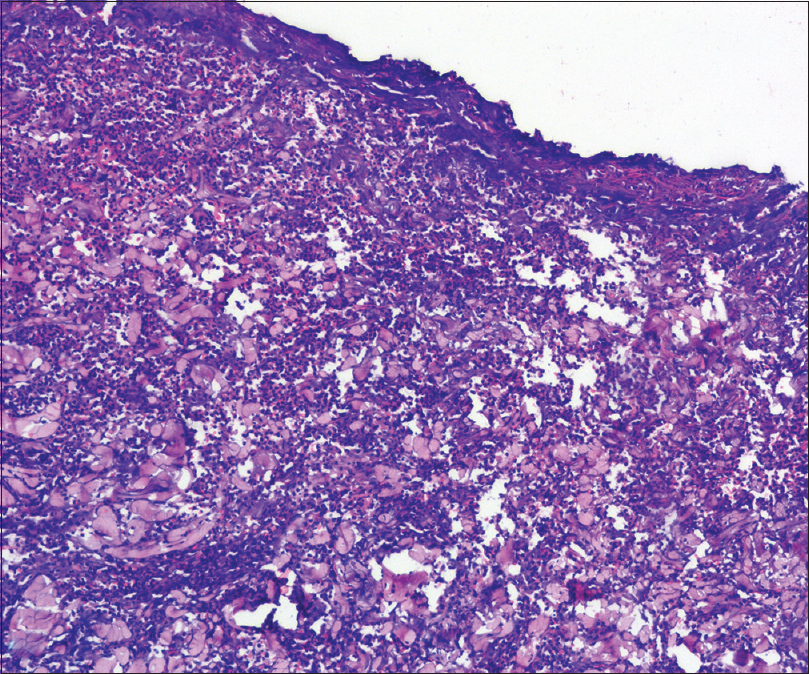 |
| Figure 2: Epidermal ulceration and extensive neutrophilic infiltration of the dermis forming multiple microabscesses (H and E, ×100) |
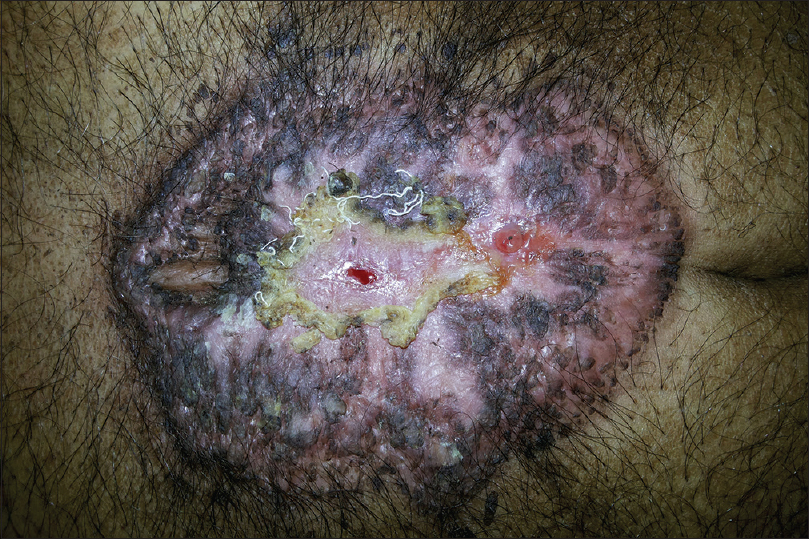 |
| Figure 3: Almost complete healing of the lesion after two months of treatment with cyclosporine |
Pyoderma gangrenosum is an inflammatory disorder characterized by sterile neutrophilic infiltration of the skin. It can clinically manifest as ulcerative, pustular, bullous, vegetative, peristomal and postoperative pyoderma gangrenosum.[1] Postoperative pyoderma gangrenosum is the consequence of pathergy phenomenon at the surgical incision site in the immediate postoperative period.[2] Its onset follows a sequence of normal evolution of scar following a surgical procedure, followed by the development of small foci of dehiscence, which progress to larger areas of wound ulceration with violaceous and undermined borders.[3] Patients usually have associated fever and malaise. In a systematic review of 220 cases, it was most commonly found after breast surgery (25%). Abdominal, cardiothoracic, orthopedic, obstetric and gynecologic or head and neck surgery were among the other causes.[4] The average latent period is usually seven days from the day of surgery, but can be earlier in obstetric, gynecologic and head and neck surgeries. The risk factors for the development of this condition are a previous history of pyoderma gangrenosum, inflammatory bowel disease, rheumatoid arthritis and hematologic disorders such as leukemia, lymphoma or myelofibrosis. Our case had a previous history of pyoderma gangrenosum. Misdiagnosis of postoperative pyoderma gangrenosum as a wound infection leads to increased morbidity due to the lack of response to antibiotics and exacerbation with improper debridement.
Post-operative pyoderma gangrenosum should be treated with systemic prednisone and other agents as like cyclosporine, cyclophosphamide, methotrexate, dapsone, and clofazimine as appropriate.[3],[5] Surgical debridement should be avoided. Empirical treatment with oral prednisolone in a dose of 1 mg/kg/day, intravenous methylprednisolone 0.5 to 1 mg/kg/dose or cyclosporine 3–5 mg/kg/day leads to prompt improvement of the wound and the general condition of the patient within 24 hours.[4] Corticosteroids or other immunosuppressive agents should be cautiously tapered only after stabilization of the wound, which usually takes two to three weeks. They should be continued until the complete healing of the wound. Medical management frequently results in a protracted course with slow wound healing, requiring a mean of 11.5 ± 11.1 months to induce disease remission.[4] In our case, we managed the patient with cyclosporine and avoided corticosteroids as the patient was diabetic and had a gastrojejunostomy. Two patients of postoperative pyoderma gangrenosum following breast surgery have been successfully treated with oral cyclosporine (2.5–5 mg/kg/day). The lesions healed completely without recurrence over a 16 to 22-week period.[6] Post-operative pyoderma gangrenosum following abdominoplasty was effectively treated with cyclosporine, following which the wounds were successfully repaired using split-thickness skin grafts under peri-operative coverage of cyclosporine.[7] A case of postoperative pyoderma gangrenosum following breast reconstruction was effectively treated with cyclosporine 300 mg/day. The wounds showed signs of healing within two weeks and healed completely over a three-month period.[5] During the period of quiescence, skin grafting or flap repair can be performed under steroid cover. Prophylactic steroids or immunomodulators such as cyclosporine or azathioprine are advocated during surgery, and tapered gradually over six months to prevent recurrence.[4] The dose of cyclosporine was increased from the maintenance dose of 100 mg/day to 5 mg/kg/day before orthopedic surgeries to prevent development of post operative pyoderma gangrenosum.[8] Successful split-thickness skin grafts are possible only after the stabilization of the post-operative pyoderma gangrenosum wound. Nine out of twelve split-thickness skin grafts done for post-operative pyoderma gangrenosum after breast surgery were successful only after immunosuppressive therapy.[9] Split-thickness skin grafts have been successfully performed for large pyoderma gangrenosum ulcers after disease control with 1 mg/kg/day prednisone.[10] It is advocated that skin grafts can be performed after the control of initial inflammatory and necrotic phases with sufficiently high doses of steroids. The high dose is to be continued throughout the surgery and tapered over a six month period to prevent recurrence. Oral cyclosporine in a dose of 3–5 mg/kg/day had been successfully used for 4–9 months before grafting with split-thickness skin grafts.[8] Some recommend subcuticular sutures to minimize the risk of post-operative pyoderma gangrenosum as this method avoids puncturing the skin surface.[11] Moist wound management is effective in promoting the wound healing. Foam dressings or laminate dressings composed of different layers are recommended due to the presence of heavy exudate.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Wollina U. Pyoderma gangrenosum – A review. Orphanet J Rare Dis 2007;2:19. [Google Scholar] |
| 2. | Faghihi G, Abtahi-Naeini B, Nikyar Z, Jamshidi K, Bahrami A. Postoperative pyoderma gangrenosum: A rare complication after appendectomy. J Postgrad Med 2015;61:42-3. [Google Scholar] |
| 3. | Ouazzani A, Berthe JV, de Fontaine S. Post-surgical pyoderma gangrenosum: A clinical entity. Acta Chir Belg 2007;107:424-8. [Google Scholar] |
| 4. | Zuo KJ, Fung E, Tredget EE, Lin AN. A systematic review of post-surgical pyoderma gangrenosum: Identification of risk factors and proposed management strategy. J Plast Reconstr Aesthet Surg 2014;29:1-9. [Google Scholar] |
| 5. | MacKenzie D, Moiemen N, Frame JD. Pyoderma gangrenosum following breast reconstruction. Br J Plast Surg 2000;53:441-3. [Google Scholar] |
| 6. | Schöfer H, Baur S. Successful treatment of postoperative pyoderma gangrenosum with cyclosporin. J Eur Acad Dermatol Venereol 2002;16:148-51. [Google Scholar] |
| 7. | Patel AJ, O'Broin ES. Pyoderma gangrenosum following abdominoplasty – A rare complication. Eur J Plast Surg 2007;29:317-9. [Google Scholar] |
| 8. | Zakhireh M, Rockwell WB, Fryer RH. Stabilization of pyoderma gangrenosum ulcer with oral cyclosporine prior to skin grafting. Plast Reconstr Surg 2004;113:1417-20. [Google Scholar] |
| 9. | Tuffaha SH, Sarhane KA, Mundinger GS, Broyles JM, Reddy SK, Azoury SC, et al. Pyoderma gangrenosum after breast surgery: Diagnostic pearls and treatment recommendations based on a systematic literature review. Ann Plast Surg 2016;77:e39-44. [Google Scholar] |
| 10. | Kaddoura IL, Amm C. A rationale for adjuvant surgical intervention in pyoderma gangrenosum. Ann Plast Surg 2001;46:23-8. [Google Scholar] |
| 11. | Long CC, Jessop J, Young M, Holt PJ. Minimizing the risk of post-operative pyoderma gangrenosum. Br J Dermatol 1992;127:45-8. [Google Scholar] |
Fulltext Views
3,360
PDF downloads
2,313





