Translate this page into:
Predicting the detection of leprosy in a hyperendemic area of Brazil: Using time series analysis
Corresponding author: Prof. Gilberto Bezerra, Aggeu Magalhães Research Center, Oswaldo Cruz Foundation, Av. Moraes do Rego, s/n, Cidade Universitária - 50670-420 Recife, Pernambuco, Brazil. gilberts@ufrn.edu.br
-
Received: ,
Accepted: ,
How to cite this article: Gregório V, Pedroza D, Barbosa C, Bezerra G, Montarroyos U, Bonfim C, et al. Predicting the detection of leprosy in a hyperendemic area of Brazil: Using time series analysis. Indian J Dermatol Venereol Leprol 2021;87:651-9.
Abstract
Background:
Brazil has the second highest prevalence of leprosy worldwide. Autoregressive integrated moving average models are useful tools in surveillance systems because they provide reliable forecasts from epidemiological time series.
Aim:
To evaluate the temporal patterns of leprosy detection from 2001 to 2015 and forecast for 2020 in a hyperendemic area in northeastern Brazil.
Methods:
A cross-sectional study was conducted using monthly leprosy detection from the Brazil information system for notifiable diseases. The Box–Jenkins method was applied to fit a seasonal autoregressive integrated moving average model. Forecasting models (95% prediction interval) were developed to predict leprosy detection for 2020.
Results:
A total of 44,578 cases were registered with a mean of 247.7 cases per month. The best-fitted model to make forecasts was the seasonal autoregressive integrated moving average ((1,1,1); (1,1,1)). It was predicted 0.32 cases/100,000 inhabitants to January of 2016 and 0.38 cases/100,000 inhabitants to December of 2020.
Limitations:
This study used secondary data from Brazil information system for notifiable diseases; hence, leprosy data may be underreported.
Conclusion:
The forecast for leprosy detection rate for December 2020 was < 1 case/100,000 inhabitants. Seasonal autoregressive integrated moving average model has been shown to be appropriate and could be used to forecast leprosy detection rates. Thus, this strategy can be used to facilitate prevention and elimination programmes.
Keywords
Communicable disease control
epidemiology
leprosy
neglected diseases
time series analysis
Introduction
Leprosy is a chronic infection caused by Mycobacterium leprae. It is a neglected disease that affects mainly developing countries constituting a severe global public health problem.1 In 2016, 214,783 new cases were recorded with a detection rate worldwide of 0.23 cases per 10,000 inhabitants.2
In 1991, the World Health Organization established leprosy elimination as a public health goal aiming to reduce the prevalence to <1 case per 10,000 inhabitants at global level by 2000. The new leprosy elimination goals include interruption of global transmission or full elimination by 2020, followed by a reduction of grade 2 disability in detected cases to less than one case per million people by 2020.2
Although combined worldwide efforts to achieve leprosy elimination goals have contributed to decreasing the number of cases during the past decade, more than 200,000 new cases are still reported every year. Brazil, India and Indonesia were responsible for 81% of them, reporting over 162,000 cases.2
Brazil has the second highest prevalence of leprosy worldwide.2 In 2016, 25,218 new cases were recorded, leading to a prevalence of 1.1 cases/10,000 inhabitants.3 The highest detection and prevalence rates were concentrated in the northern, northeastern and central-western regions of the country. The state of Pernambuco was classified as “high” prevalence because of its general detection rate of 19.7 cases per 100,000 inhabitants. Moreover, among 1856 new cases, 1571 were evaluated for disability grade with 82 of them (5.2%) presenting with grade 2 disability.4
Time series forecasts regarding diseases and adverse health conditions are some of the most important applications in epidemiology such as detection and prevalence rates, especially concerning surveillance and effective planning of healthcare actions.5,6 Time series studies have been widely applied in the field of healthcare. One of the main objectives of public health surveillance systems is to provide reliable forecasts from epidemiological time series.7 Autoregressive integrated moving average models are useful tools for modeling time series regarding trends, cyclicity and seasonality.8-10
The autoregressive integrated moving average model seeks time dependence among successive observations11 and has been widely used in studies on transmitted diseases.6,12-14 Autoregressive integrated moving average models have been successfully applied for forecasting the incidence of infectious diseases, for example, morbidity due to influenza,15 the incidence of malaria16 and dengue12 as well as the prevalence of cutaneous leishmaniasis.17 In this study, it was sought to use a time series analysis model to predict leprosy detection in a hyperendemic area. The study aimed to evaluate the temporal patterns of leprosy detection from 2001 to 2015 and do a forecast for 2020 in a state of northeastern Brazil.
Methods
Study area
The state of Pernambuco (8° 19’ 59” S; 37° 45’ 0” W) is located in the northeastern region of Brazil. The estimated population in 2017 was 9,473,266 and over 80% lived in urban areas. The state’s population density is 89.62 inhabitants/km2 living in an area of 98,076,021 km2. The state of Pernambuco is organized into five mesoregions, 12 health regions and 185 municipalities which are the smallest administrative unit. Regarding social indicators, the state presents a human development index of 0.673 (18th in the country’s ranking), and Gini index of 0.62, such that 12.3% of the population live in extreme poverty and 46.7% of the population that live in private dwellings have inadequate basic sanitation services.18
Study design
This was an ecological study with time-trend analysis. The time unit used in this study was “month.” The monthly case series created 180 data points between January 2001 and December 2015. The municipality was the unit used in spatial analysis.
Data source and study variables
The study population consisted of new leprosy cases registered in the Brazil information system for notifiable diseases. They were individuals who had never received treatment for the infection, living in the state of Pernambuco, diagnosed between January 1st, 2001 and December 31st, 2015.
Leprosy case notification occurred after diagnosis and treatment in primary health care units in Pernambuco state. Patients presenting with grade 2 disability or reactions to the treatment were referred to one of the 12 reference units in the state.
Leprosy data was extracted from Brazil information system for notifiable diseases, through the health department of the state of Pernambuco. Brazil information system for notifiable diseases is an epidemiological surveillance system within the Brazilian healthcare system that provides information on diseases and adverse health conditions in Brazil for which notification is compulsory.
Brazil information system for notifiable diseases data is composed of compulsory notification records that consist of standardized forms in which sociodemographic and clinical information is filled in by healthcare professionals. In this study, records that presented errors of diagnosis or were duplicated were excluded.
Population-based data was obtained from the Brazilian institute of geography and statistics and were used to calculate leprosy detection rates over the period of study.
The variables selected in this study were: sex, race/color, educational level, age, municipality of residence, zone of residence, clinical form (indeterminate, tuberculoid, borderline or lepromatous), operational classification (paucibacillary or multibacillary), number of skin lesions and disability grade at the time of the diagnosis (0, I or II). The variable “educational level” could not be analyzed because a high proportion of the records did not provide this information.
Statistical analysis
Exploratory analysis of the data was conducted using the R software version 3.5.1. Absolute and relative frequencies were calculated for categorical variables.
Autoregressive integrated moving average model
The temporal patterns of leprosy detection rates per month for the study period were analyzed and the general characteristics of the data were evaluated using this graphical approach: trends (increase, decrease), seasonality, outliers and smooth changes in structure.19-22
The Box–Jenkins approach was used to fit autoregressive integrated moving average models which are defined by three terms (p, d, q) and used for nonseasonal time series. The first step of the model identification was to evaluate the trend component (d). We explored the monthly detection of leprosy cases with 12 months of periodicity (S = 12 observations per year). The series was transformed by differencing the scores (months) to make it stationary, if appropriate. The number of differencing operations is the d parameter. As a second step, the autoregressive component (value of p) was identified. As a third step, the moving average component, value of q was identified.
The seasonal component was included using seasonal autoregressive integrated moving average model if the previous analysis indicated evidence of seasonality. This component has three more parameters denoted P, D and Q. These parameters are similar to p, d and q, but operate on the scale of the periodicity (12 months). Analysis of the shape of the autocorrelation functions and partial autocorrelation functions allowed estimation of the autoregressive and moving average parameters and identification of plausible models.
Forecasts for future value in the series were made using an equation for which parameters were obtained employing maximum likelihood. The database used for estimating the parameters of the model consisted of the data available on the series analyzed. For forecasting purposes, the estimated equation is assumed to be the series generating process.
For the initial stage in constructing, an autoregressive integrated moving average (or seasonal autoregressive integrated moving average) model is needed to specify which treatment (p, q, d or P, Q, D) should be attributed to a series in order to estimate parameters. To choose these arguments, the criteria established in the literature have to be followed.8-10,12
First, a graph depicting series evolution over time is viewed in order to see whether any type of pattern is visible (stationarity, trend, seasonality and other). In addition to visual analysis, statistical tests such as correlograms and Dickey–Fuller tests are conducted to identify whether any stationarity is present. For forecasts, the series should be stationary. Therefore, if nonstationarity is identified in a forecast, it is appropriate to differentiate the series to search for a more stationary pattern. Finally, statistical tests are useful for defining seasonality patterns in the series.
Cyclical behavior is processed using the moving average procedure. Autocorrelation functions and partial autocorrelation functions are statistical tests that indicate the autoregressive and moving average components of the model.12
To determine the patterns that best described the leprosy time series, the Box–Jenkins approach was followed to select the autoregressive integrated moving average model based on three stages. The detection of leprosy was plotted against time to detect and correct any nonstationarity of the time series. This showed the mean autoregressive and moving terms that were necessary for calculating autocorrelation functions and partial autocorrelation functions. In the present study, the time series for the detection rate regarding new leprosy cases per 100,000 inhabitants was analyzed. All of the statistical treatment was conducted using the time-series forecasting packages of the R software.
Ethical statement
This study was approved by the Research Ethics Committee of the University of Pernambuco (CAAE: 60748116.0.0000.5207). All data analyzed was anonymized.
Results
Epidemiological profile of the cases
A total of 44,578 cases were recorded, of whom the majority were women (51.4%), older than 15 years of age (89.4%) and living in urban areas (89.4%). The clinical forms more common were borderline (31.5%) and tuberculoid (31.3%). In addition, 59.7% of the cases displayed up to five lesions and 5.2% presented grade 2 disability [Table 1].
| Variables | Number of cases (%) (total=44,578) |
|---|---|
| Sex: Female | 22,928 (51.4) |
| Age (years) | |
| <15 | 4708 (10.6) |
| ≥15 | 39,870 (89.4) |
| Duration of formal education (years) | |
| 1-5 | 10,675 (35.5) |
| 6-9 | 11,140 (37.5) |
| 10-12 | 4419 (14.7) |
| >12 | 3828 (12.7) |
| No information | 14,516 |
| Zone of residence | |
| Urban | 36,389 (89.4) |
| Rural | 4073 (10.0) |
| Periurban | 219 (0.6) |
| No information | 3897 |
| Clinical form | |
| Undetermined | 9090 (22.5) |
| Tuberculoid | 12,642 (31.3) |
| Borderline | 12,762 (31.5) |
| Lepromatous | 5960 (14.7) |
| Not classified | 2227 |
| No information | 1897 |
| Clinical form | |
| Undetermined | 22,650 (51.0) |
| Tuberculoid | 21,763 (49.0) |
| No information | 165 |
| Number of lesions | |
| No lesion | 7259 (16.7) |
| Up to 5 lesions | 25,947 (59.7) |
| 6 or more lesions | 10,284 (23.6) |
| No information | 1088 |
| Disability grade | |
| Zero | 30,858 (77.0) |
| Grade I | 7136 (17.8) |
| Grade II | 2100 (5.2) |
| Not evaluated | 3471 |
| No information | 1013 |
Figure 1 shows the distribution of municipalities according to the prevalence rates per 5-year period. Figure 1a shows that in the first 5 years, nine municipalities had a zero-detection rate, while 42 were hyperendemic areas. For the second 5-year period [Figure 1b], two municipalities had a zero rate with little variation in the number of hyperendemic municipalities, 45 areas. In the last 5 years analyzed, four municipalities had a detection rate of zero and 43 were considered hyperendemic municipalities [Figure 1c]. Among the three periods of 5 years analyzed, the highest detection rates were found in the municipalities located in the metropolitan region of Recife.
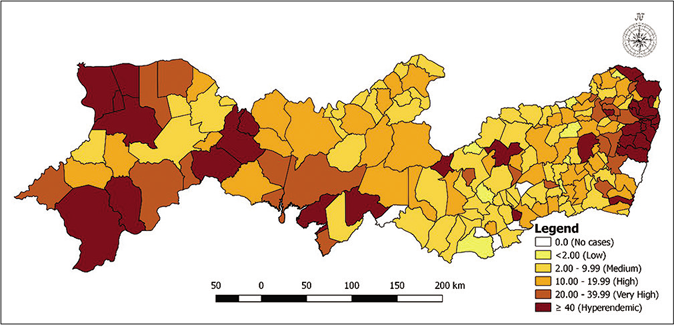
- Distribution of municipalities according to detection rates (per 100,000 inhabitants) per 5-year period in Pernambuco, Brazil: 2001–2005
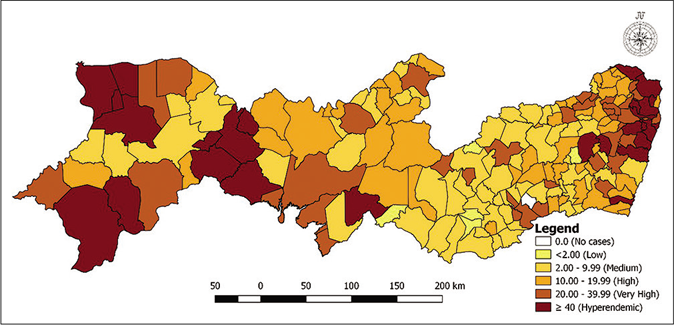
- Distribution of municipalities according to detection rates (per 100,000 inhabitants) per 5-year period in Pernambuco, Brazil: 2006–2010
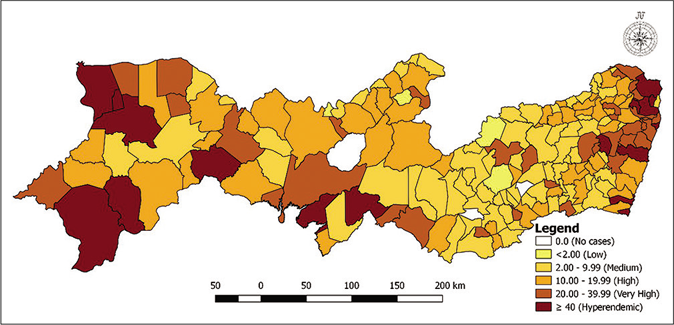
- Distribution of municipalities according to detection rates (per 100,000 inhabitants) per 5-year period in Pernambuco, Brazil: 2011–2015
Autoregressive integrated moving average model
The total number of leprosy cases during the period studied was 44,578 with a mean of 247.7 cases per month. The number of confirmed cases per month ranged from 134 to 411. The mean detection rate for the period was 2.9, ranging from 1.5 to 5.0. The number of multibacillary cases during the period was 21,763 with a mean rate of 1.4 (0.7 to 2.1). A total of 2100 grade 2 disability cases were notified at the time of diagnosis with a mean of 11.7 cases per month (3–24 cases). A declining trend and the existence of seasonal variations in detection rates for new cases were observed [Figure 2a].

- Detection rate for new cases of leprosy (per 100,000 inhabitants)
This series was nonstationary; in other words, it did not provide constant mean and variance values. Figure 2b presents the decomposition of the series into its constituent elements (trend and seasonality). Regarding the detection rate for new cases, a declining trend for the series with the seasonal component could be observed. The series decomposition into its elements, namely, trend and seasonal component enabled a more precise analysis. A clear declining trend in the second part of the series could be observed. In the first part of the figure, the original series is reproduced. In turn, the seasonal component is very evident in the third part of the figure.

- Trend, seasonal and residual components derived from the time series decompositions for the monthly detection rate regarding new cases of leprosy
The correlogram shows the relationship between the covariance/variance of the series and its lags in k periods. A series is considered to be stationary when its correlogram shows mild fluctuations around zero. Figure 2c shows that none of the bars for lags of up to 12 periods (i.e., those considered in this test) were within the interval that would have suggested that the series was stationary. This indicates that the series needed to be transformed for forecasting purposes.

- Autocorrelation function correlogram for the detection rate regarding new cases of leprosy. Pernambuco, Brazil, between 2001 and 2015
Figure 3 shows more clearly that seasonality in the detection rate for new cases of leprosy was occurring. The variation from the straight lines that denoted the mean monthly values for the years considered in this study showed that the series had seasonal behavior. For the time interval considered, i.e., between 2001 and 2015, the lowest numbers of occurrences were seen to be in January and December.
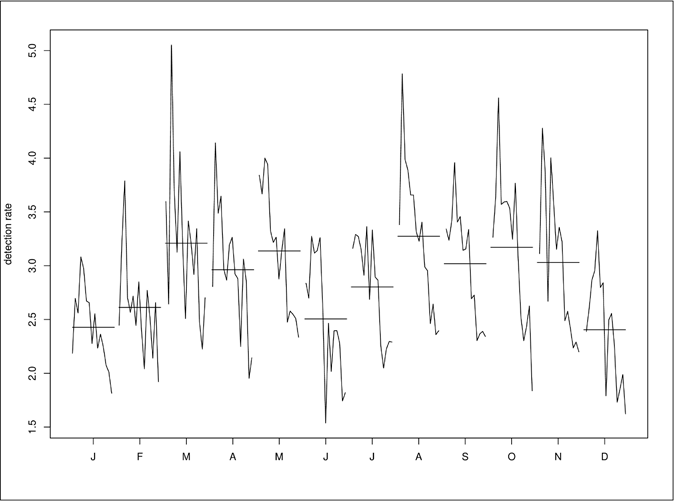
- Seasonal variation in the overall detection rate for leprosy (per 100,000 inhabitants), in Pernambuco, Brazil, between 2001 and 2015
Statistical evidence regarding stationarity is also produced through the Dickey–Fuller test. In this test, the null hypothesis (H0) is that the series presents a unitary root and is not stationary. The alternative hypothesis (H1) states that the root is negative and the series is stationary.
The results from this test for the series of leprosy cases showed that the calculated value from the test statistics (tau statistics) had a lower modulus than the critical values (for the 90, 95 and 99% confidence levels). Therefore, the hypothesis H0 had to be accepted and, thus, the series could be defined as not stationary. The most usual procedure for seeking stationarity in a nonstationary time series consists of differentiation: the value for one date minus the value of the previous date.
To make forecasts, the autoregressive integrated moving average model was used with seasonal component modeling, i.e., seasonal autoregressive integrated moving average. Various combinations were tested and the seasonal autoregressive integrated moving average model ((1,1,1); (1,1,1)) was reached for the calculation of the autoregressive, integrated and moving mean components, both for the nonseasonal and the seasonal parts of the model. Figure 4 shows the original data series (in red) and the series estimated through the model (in blue).

- Comparison between observed monthly time series and the series estimated by seasonal autoregressive integrated moving average, regarding the detection rate for new cases of leprosy
Figure 5 presents the forecasting results for the series regarding the detection rate for new cases of leprosy. The gray shadow indicates the 95% confidence interval for the forecasts that were made.
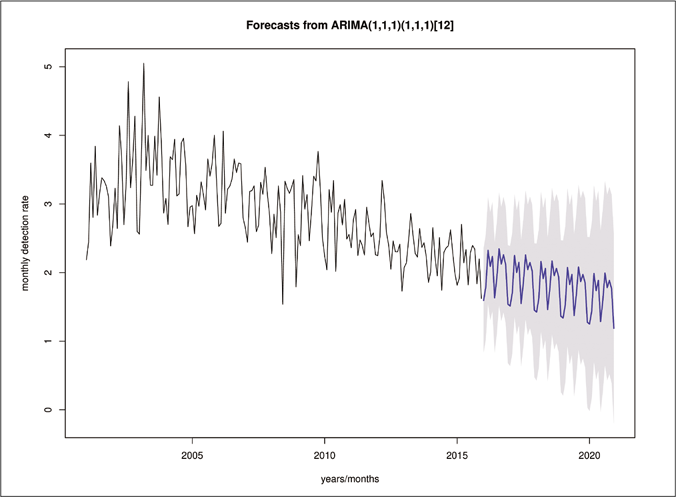
- Forecasted values for the detection rate of leprosy new cases are shown by the blue line while shadowed areas show the 95% confidence interval
Table 2 shows the monthly forecast of the leprosy detection rate per 100,000 inhabitants. According to the model, with 95% forecast range (95% PI), the detection rate ranged from 0.32 cases/100,000 inhabitants in January of 2016 to 0.38 cases/100,000 inhabitants in December of 2020.
| Period | PIR (95% CI) |
|---|---|
| January 01, 2016 | 0.32 (0.17-0.46) |
| February 01, 2016 | 0.34 (0.18-0.51) |
| March 01, 2016 | 0.33 (0.13-0.53) |
| April 01, 2016 | 0.33 (0.10-0.55) |
| May 01, 2016 | 0.35 (0.10-0.59) |
| June 01, 2016 | 0.31 (0.03-0.58) |
| July 01, 2016 | 0.31 (0.02-0.60) |
| August 01, 2016 | 0.36 (0.05-0.67) |
| September 01, 2016 | 0.33 (0.00-0.66) |
| October 01, 2016 | 0.33 (−0.00-0.68) |
| November 01, 2016 | 0.34 (−0.02-0.70) |
| December 01, 2016 | 0.34 (−0.03-0.72) |
| January 01, 2017 | 0.32 (−0.07-0.71) |
| February 01, 2017 | 0.34 (−0.07-0.75) |
| March 01, 2017 | 0.32 (−0.09-0.75) |
| April 01, 2017 | 0.32 (−0.11-0.76) |
| May 01, 2017 | 0.36 (−0.09-0.81) |
| June 01, 2017 | 0.30 (−0.16-0.77) |
| July 01, 2017 | 0.31 (−0.17-0.79) |
| August 01, 2017 | 0.36 (−0.13-0.85) |
| September 01, 2017 | 0.34 (−0.17-0.84) |
| October 01, 2017 | 0.35 (−0.17-0.87) |
| November 01, 2017 | 0.35 (−0.19-0.88) |
| December 01, 2017 | 0.35 (−0.19-0.90) |
| January 01, 2018 | 0.33 (−0.23-0.89) |
| February 01, 2018 | 0.35 (−0.23-0.93) |
| March 01, 2018 | 0.33 (0.26-0.93) |
| April 01, 2018 | 0.33 (−0.27-0.94) |
| May 01, 2018 | 0.36 (−0.25-0.99) |
| June 01, 2018 | 0.31 (−0.32-0.95) |
| July 01, 2018 | 0.32 (−0.33-0.97) |
| August 01, 2018 | 0.36 (−0.29-1.04) |
| September 01, 2018 | 0.34 (−0.34-1.03) |
| October 01, 2018 | 0.35 (−0.34-1.05) |
| November 01, 2018 | 0.35 (−0.35-1.07) |
| December 01, 2018 | 0.36 (−0.36-1.09) |
| January 01, 2019 | 0.34 (−0.40-1.08) |
| February 01, 2019 | 0.35 (−0.40-1.12) |
| March 01, 2019 | 0.34 (−0.43-1.12) |
| April 01, 2019 | 0.34 (−0.44-1.13) |
| May 01, 2019 | 0.37 (−0.43-1.18) |
| June 01, 2019 | 0.32 (−0.50-1.14) |
| July 01, 2019 | 0.33 (−0.51-1.17) |
| August 01, 2019 | 0.38 (−0.47-1.23) |
| September 01, 2019 | 0.36 (−0.51-1.22) |
| October 01, 2019 | 0.36 (−0.52-1.25) |
| November 01, 2019 | 0.37 (−0.53-1.26) |
| December 01, 2019 | 0.37 (−0.54-1.28) |
| January 01, 2020 | 0.35 (−0.58-1.28) |
| February 01, 2020 | 0.37 (−0.58-1.32) |
| March 01, 2020 | 0.35 (−0.61-1.32) |
| April 01, 2020 | 0.35 (−0.63-1.34) |
| May 01, 2020 | 0.38 (−0.61-1.38) |
| June 01, 2020 | 0.33 (−0.68-1.34) |
| July 01, 2020 | 0.34 (−0.69-1.37) |
| August 01, 2020 | 0.39 (−0.66-1.44) |
| September 01, 2020 | 0.37 (−0.69-1.43) |
| October 01, 2020 | 0.37 (−0.70-1.46) |
| November 01, 2020 | 0.38 (−0.72-1.47) |
| December 01, 2020 | 0.38 (−0.73-1.49) |
PIR: Prediction interval, 95% CI: 95% confidence interval
Discussion
The results from this study regarding the epidemiological characteristics of new leprosy cases showed that most of the patients were women, older than 15 years of age and living in urban areas. Leprosy affects both sexes, but the proportion of cases among men is higher than among women,23,24 at a ratio of between 1.5 and 2.25 In Dhaka, Bangladesh, a male-to-female ratio of 5:4 was found.24 In Brazil, a study that evaluated the epidemiological profile of leprosy over 20 years showed higher detection among men, and an increase in the male-to-female ratio to 1.09 by the end of the period studied.26 Results similar to those obtained in this study, with a higher occurrence among women, were identified in other Brazilian studies.27-29 A population cohort study on the incidence of leprosy with total of 68 cases showed a proportion of 60% (41 cases) among women, but the incidence rate was similar for both sexes.30
Regarding the age group, individuals older than 15 years of age were predominant. Leprosy occurs more frequently among adults because of the long incubation period; however, the proportion of the cases among individuals younger than 15 years old in this study was 24% (16 cases)30 which is higher than a study done in Brazil with a proportion of 7.5%.31
The occurrence of leprosy in individuals younger than 15 years of age is indicative of early exposure, reflects the intensity of propagation of the infecting agent and it is important for monitoring the epidemiological impact of control programmes that are implemented.32 A survey analyzed the clinical, bacteriological and histopathological characteristics of newly detected cases of childhood leprosy in the community and found that most cases were paucibacillary, had at least one lesion and the average duration of symptoms exceed 1 year. This indicates the severity of the problem in the failure to detect childhood leprosy, recent active transmission, poor knowledge about the disease and barriers to accessing or using health care.33
The borderline and tuberculoid clinical forms and the multibacillary operational classification prevailed in this study. After long exposure to Mycobacterium leprae, only a subgroup of exposed individuals develops clinical leprosy and this presents a wide spectrum of clinical manifestations that range from paucibacillary to multibacillary forms.1 The Brazilian ministry of health considers the tuberculoid and indeterminate clinical forms of leprosy to be paucibacillary (up to five skin lesions) and the borderline and/or lepromatous form to be multibacillary (presence of six or more skin lesions).34 Leprosy classification is important for determining prognosis and implementing adequate treatment for the disease, and findings of multibacillary cases indicate late diagnosis and flaws in leprosy elimination programmes.
Currently, multibacillary patients constitute 60.2% of new cases detected globally. As global leprosy numbers decline, the percentage of multibacillary leprosy cases, a proportion with high initial bacterial burden, is likely to increase further in the coming years as expected in the epidemiological pattern of a declining disease. Therefore, it is imperative to approach and plan multibacillary leprosy therapy carefully.35
This study focused on a time series of detection rates and forecasted the trend for the disease in the future by using monthly detection data. The results revealed that the seasonal autoregressive integrated moving average model ((1,1,1); (1,1,1)) adequately forecasted the disease trend in the study area. According to the result of the model, the detection rate followed a declining trend with a seasonal component. Besides, the adjusted numbers in the model and the normal distribution of residuals showed that this model provided a good fit.
The detection rate for new cases of leprosy in the state of Pernambuco was seen to increase in March (austral autumn) and August (austral winter), and decrease in December and January (austral summer). Leprosy is traditionally not acknowledged as a seasonal disease. The first survey that analyzed the impact of seasonality on the detection rate of new leprosy cases in Brazil showed that in the north, northeast, center-west and southeast, there were statistically significant variations almost every month of the year. The largest increases were observed in March and May (autumn) and August (winter) and the largest decreases were observed in December (summer).36 In this study, a reduced detection rate was also observed in June. Similarly, the study also identified a reduced detection rate in the same month.36 It is possibly associated with the region’s holiday season, including festivals in honor of patron saints that supposedly influence the dynamics of health service providers because they are large-scale tourist events. It is noteworthy that results related to seasonality may also be related to health care. Hence, information about seasonality can be used to define strategies for finding new cases by health surveillance services.
A study on dermatological hospitalizations analyzed the profiles of admissions associated with the seasons of the year and showed that there was a higher incidence of leprosy.37 A study that evaluated the presence of M. leprae in the nasal mucosa of the population identified seasonal variation and a possible association among environmental conditions (climate), the transmission patterns and levels of exposure to M. leprae.38 In order to assure success in leprosy elimination program actions, it is necessary to understand the geographical distribution of the disease, its natural history and pathogen-host interactions.37
The results from this study showed that there was seasonal variation in the detection of leprosy and that climate changes may influence transmission. It would be suggested that the seasonal patterns of leprosy need to be taken into account in planning control actions and determining the magnitude of healthcare service provision. Acknowledgment of seasonality is important for developing effective surveillance strategies in endemic geographical regions.36
The trend analysis of leprosy detection rates performed in this study showed declining behavior. Time series analyses using different international and national models have shown a reduction in leprosy detection rates.39-42 Morocco has achieved the goal of leprosy elimination as a public health problem several years ago, with a downward trend from 2000 to 2017.41 In the Republic of Korea, there has been a reduction in prevalence and incidence rates in recent decades and M. leprae transmission may have effectively ceased.43
In Brazil, studies conducted in the states of Maranhão, Sergipe and Tocantins as well as the city of Fortaleza showed a reduction in case detection rates.39,40,44 Although the results of these studies displayed a reduction in leprosy’s detection, its transmission persists in these areas. Socioeconomic and living factors probably play a relevant role in maintaining endemicity. Programs aimed at eliminating leprosy as a public health problem should include interventions that also reduce social inequality.42 For the success of the leprosy elimination strategy, it is necessary to consider, in addition to diagnostic technologies and multidrug therapy in health services, essential factors for human development and well-being.29,35
In this study, different seasonal autoregressive integrated moving average models were evaluated and the best one was chosen. Forecasting of disease trends is an essential issue in early epidemic alert systems and is paramount for disease prevention and for planning control actions relating to transmitted diseases. In diseases with seasonal trends, the seasonal autoregressive integrated moving average model can be used to adequately predict trends by eliminating the seasonal component.
Limitations
There are a few limitations to this study. First, data from Brazil information system for notifiable diseases were used and there is the possibility of underreporting which could decrease leprosy detection rate. These undetected cases may have influenced the detection rates, consequently, the indicators and the trend detected in the study; nevertheless, this system is the main Brazilian source of information on transmitted diseases. Other factors, such as temperature, humidity, socioeconomic status and access to healthcare services may also influence the transmission of leprosy and should be examined in future studies.
Conclusion
Objective of this study was to forecast the detection of leprosy. Monthly detection data covering 15 years were used to investigate the best model which was found to be seasonal autoregressive integrated moving average ((1,1,1); (1,1,1)). The detection of leprosy has demonstrated a seasonal behavior and the autoregressive integrated moving average model was found to be appropriate to forecast leprosy detection rates. Thus, this strategy can be used to facilitate efforts in the prevention and elimination of the disease.
Acknowledgment
The authors thank the Health Department of the State of Pernambuco for Leprosy data provided through the Brazil information system for notifiable diseases (SINAN).
Financial support and sponsorship
This work was supported by the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) (grant number IBPG-0397-4.01/17) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (grant number 001).
Conflicts of interest
There are no conflicts of interest.
References
- Pauci-and multibacillary leprosy: Two distinct, genetically neglected diseases. PLoS Negl Trop Dis. 2016;10:e0004345.
- [Google Scholar]
- Global Leprosy Update, 2016: Accelerating Reduction of Disease Burden. 2017. Geneva: WHO; Available from: https://www.who.int/lep/resources/who_wer9235/en/ [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Secretaria de Vigilância em Saúde Caracterização da Situação Epidemiológica da Hanseníase e diferenças por sexo Brasil 2012-2016. Bol Epidemiol. 2018;19:1-12.
- [Google Scholar]
- Ministério da Saúde, Leprosy Prevalence Rate 1990 to 2017. 2017. Brasília: Ministério da Saúde. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2018/julho/13/Taxa-de-prevalencia-dehanseniase-1990a2017.pdf [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Use of time-series analysis in infectious disease surveillance. Bull World Health Organ. 1998;76:327-33. Available from: https://apps.who.int/iris/handle/10665/55936 [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Temporal and long-term trend analysis of class C notifiable diseases in China from 2009 to 2014. BMJ Open. 2016;6:e011038.
- [Google Scholar]
- Dynamic linear model and SARIMA: A comparison of their forecasting performance in epidemiology. Stat Med. 2001;20:3051-69.
- [CrossRef] [Google Scholar]
- Modeling and forecasting of the under-five mortality rate in Kermanshah province in Iran: A time series analysis. Epidemiol Health. 2015;37:e2015003.
- [Google Scholar]
- Seasonality of active tuberculosis notification from 2005 to 2014 in Xinjiang, China. PLoS One. 2017;12:e0180226.
- [Google Scholar]
- Time series analysis of the association between ambient temperature and cerebrovascular morbidity in the elderly in Shanghai, China. Sci Rep. 2016;6:19052.
- [CrossRef] [Google Scholar]
- Time Series Analysis and Forecasting Switzerland: Springer International Publishing; 2016.
- [Google Scholar]
- Time series analysis of dengue surveillance data in two Brazilian cities. Acta Trop. 2018;182:190-7.
- [CrossRef] [Google Scholar]
- Epidemiology and ARIMA model of positive-rate of influenza viruses among children in Wuhan, China: A nine-year retrospective study. Int J Infect Dis. 2018;74:61-70.
- [CrossRef] [Google Scholar]
- Spatial and temporal relationships between human and canine visceral leishmaniases in Belo Horizonte, Minas Gerais, 2006-2013. Parasit Vectors. 2018;11:372.
- [CrossRef] [Google Scholar]
- Epidemiological features and forecast model analysis for the morbidity of influenza in Ningbo, China, 2006-2014. Int J Environ Res Public Health. 2017;14:e559.
- [Google Scholar]
- A weather-based prediction model of malaria prevalence in Amenfi West District, Ghana. Malar Res Treat. 2017;2017:7820454.
- [CrossRef] [Google Scholar]
- Modeling zoonotic cutaneous leishmaniasis incidence in central Tunisia from 2009-2015: Forecasting models using climate variables as predictors. PLoS Negl Trop Dis. 2017;11:e0005844.
- [Google Scholar]
- Ministério do Planejamento Desenvolvimento e Gestão (BR) Instituto Brasileiro de Geografia e Estatística. 2010. Censo Demográfico, Rio de Janeiro: IBGE. Available from: https://cidades.ibge.gov.br/brasil/pe/panorama [Last accessed on 2010 Aug 10]
- [Google Scholar]
- Disease management with ARIMA model in time series. Einstein (Sao Paulo). 2013;11:128-31.
- [CrossRef] [Google Scholar]
- Almost everything you need to know about time series. 2019. Medium. Available from: https://towardsdatascience.com/almost-everything-you-need-to-know-about-time-series-860241bdc578 [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Clinical-epidemiological profile of patients living with leprosy. Int Arch Med. 2015;8:1-9.
- [CrossRef] [Google Scholar]
- A case of lepromatous leprosy with co-existing tuberculosis verrucosa cutis (TVC) Lepr Rev. 2015;86:176-9.
- [CrossRef] [Google Scholar]
- Global Leprosy: Update on the 2012 Situation. 2013. Geneva: WHO; Available from: https://www.who.int/lep/resources/who_wer8835/en/ [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Leprosy in Southern Brazil: A twenty-year epidemiological profile. Rev Soc Bras Med Trop. 2017;50:251-5.
- [CrossRef] [Google Scholar]
- Sociodemographic and epidemiological profile of leprosy patients in an endemic region in Brazil. Rev Soc Bras Med Trop. 2016;49:777-80.
- [CrossRef] [Google Scholar]
- Clinical and epidemiological profile of leprosy patients attended at Ceará, 2007-2011. Ann Bras Dermatol. 2016;91:311-7.
- [CrossRef] [Google Scholar]
- Social determinants, their relationship with leprosy risk and temporal trends in a tri-border region in Latin America. PLoS Negl Trop Dis. 2018;12:e0006407.
- [Google Scholar]
- Leprosy incidence: Six years follow-up of a population cohort in Bangladesh. Lepr Rev. 2014;85:158-69. Available from: https://www.researchgate.net/publication/269714307_Leprosy_incidence_six_years_follow-up_of_a_population_cohort_in_Bangladesh [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Trends of main indicators of leprosy in Brazilian municipalities with high risk of leprosy transmission, 2001-2012. BMC Infect Dis. 2016;16:472.
- [CrossRef] [Google Scholar]
- Leprosy in children and adolescents under 15 years old in an urban centre in Brazil. Mem Inst Oswaldo Cruz. 2016;111:359-64.
- [CrossRef] [Google Scholar]
- Clinical, bacteriological, and histopathological characteristics of newly detected children with leprosy: A population based study in a defined rural and urban area of Maharashtra, Western India. Indian J Dermatol Venereol Leprol. 2013;79:512-7.
- [CrossRef] [Google Scholar]
- Departamento de Vigilância e Doenças Transmissíveis In: Guia Prático Sobre a Hanseníase. Brasília: Ministério da Saúde;; 2013.
- [Google Scholar]
- Global leprosy strategy 2016-2020: Issues and concerns. Indian J Dermatol Venereol Leprol. 2017;83:4-6.
- [CrossRef] [Google Scholar]
- The impact of seasonal climate on new case detection rate of leprosy in Brazil (2008-2012) Lepr Rev. 2017;88:533-42. Available from: https://www.researchgate.net/publication/324154509_The_impact_of_seasonal_climate_on_new_case_detection_rate_of_leprosy_in_Brazil_2008-2012 [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Seasonality of the hospitalizations at a dermatologic ward (2007-2017) An Bras Dermatol. 2018;93:755-8.
- [CrossRef] [Google Scholar]
- Cohort study of the seasonal effect on nasal carriage and the presence of Mycobacterium leprae in an endemic area in the general population. Clin Microbiol Infect. 2013;19:970-4.
- [CrossRef] [Google Scholar]
- Trend analysis of leprosy indicators in a hyperendemic Brazilian state, 2001-2015. Rev Saude Publica. 2019;53:61.
- [CrossRef] [Google Scholar]
- Leprosy trends in Tocantins, a hyperendemic State in the North of Brazil, 2001-2012. Cad Saude Publica. 2015;31:971-80.
- [CrossRef] [Google Scholar]
- Trend analysis of leprosy in Morocco between 2000 and 2017: Evidence on the single dose rifampicin chemoprophylaxis. PLoS Negl Trop Dis. 2018;12:e0006910.
- [Google Scholar]
- Spatial clustering, social vulnerability and risk of leprosy in an endemic area in Northeast Brazil: An ecological study. J Eur Acad Dermatol Venereol. 2019;33:1581-90.
- [CrossRef] [Google Scholar]
- The decline of leprosy in the Republic of Korea; patterns and trends 1977-2013. Lepr Rev. 2015;86:316-27. Available from: https://www.semanticscholar.org/paper/The-decline-of-leprosy-in-the-Republic-of-Korea%3B-Lee-Kim/8bc16a33a6001ca488d3007d01b5cc834c483c8c [Last accessed on 2019 Aug 10]
- [Google Scholar]
- Temporal trends of leprosy in a Brazilian state capital in Northeast Brazil: Epidemiology and analysis by join points, 2001 to 2012. Rev Bras Epidemiol. 2016;19:194-204.
- [CrossRef] [Google Scholar]






