Translate this page into:
Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms
2 Health Services, Koyilandy Taluk Hospital, Kozhikode, Kerala, India, India
3 Department of Social and Preventive Medicine, Government Medical College, Kozhikode, Kerala, India
4 General Medicine, Government Medical College, Kozhikode, Kerala, India
Correspondence Address:
Sarita Sasidharanpillai
“Rohini”, Girish Nagar, Nallalom PO, Kozhikode - 673 027, Kerala
India
| How to cite this article: Sasidharanpillai S, Chathoth AT, Khader A, Reena Mariyath OK, Riyaz N, Binitha MP, Muhammed K, George B, Santhosh P, Roslind S, Paul N, Thomas MH. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol 2019;85:266-275 |
Abstract
Background: Drug reaction with eosinophilia and systemic symptoms is an outcome of a complex interaction between specific drugs, certain herpesviruse types and the immune system of the affected individual and is characterized by an unpredictable course and recurrent flares even after withdrawal of the offending drug and administration of systemic steroids.
Aims: To identify the predictors of disease severity in drug reaction with eosinophilia and systemic symptoms.
Methods: After obtaining ethical clearance from the institutional ethics committee and a written informed consent from individual study participant, the first hundred patients who required inpatient care in Government Medical College, Kozhikode with drug reaction with eosinophilia and systemic symptoms from January 1st 2011 were included in this study aimed to identify the predictors of disease severity in drug reaction with eosinophilia and systemic symptoms.
Results: Male-to-female ratio of the study group was 0.8:1. The presence of atypical cells in peripheral smear and advanced age were found to be predictors of disease severity in drug reaction with eosinophilia and systemic symptoms, whereas, sex, facial erythema and edema and absolute eosinophil count were found not to be predictors of the same.
Limitations: The main limitation of this study was our inability to assess the role of human leukocyte antigen (HLA) association and herpes virus reactivation in disease severity in drug reaction with eosinophilia and systemic symptoms. This study was also not designed to evaluate the response to treatment given and the mortality caused by drug reaction with eosinophilia and systemic symptoms.
Conclusions: Studies on the predictors of severity in drug reaction with eosinophilia and systemic symptoms in different population groups may enable us to identify the warning signs and help to formulate the standard therapeutic guidelines.
Introduction
Since the initial description by Chaiken et al., drug reaction with eosinophilia and systemic symptoms (DRESS) has remained an enigma with its variable clinical features, close resemblance to infective, autoimmune and neoplastic diseases and unpredictable disease course.[1] Lack of a reliable diagnostic criteria or a laboratory test makes it a therapeutic challenge. One unique feature of DRESS is the waxing and waning course of reaction pattern noted in some affected patients even after withdrawal of the offending drug and administration of systemic steroids.[2],[3],[4] DRESS shows a spectrum of manifestations ranging from mild cutaneous rash to severe and life-threatening forms with major organ involvement or organ failure. No standard guidelines are available for the management of DRESS. The French Society of Dermatology has recommended a therapeutic guideline for DRESS based on internal organ involvement, organ failure and evidence of herpes virus reactivation.[5] We believe information regarding the predictors of internal organ involvement and organ failure in DRESS may help to improve the management of the affected by early identification of those individuals at risk for severe DRESS. Allopurinol and minocycline, female sex, advanced age, facial erythema, high eosinophil count, atypical lymphocytes in peripheral smear and reactivation of human herpesvirus 6 and cytomegalovirus were considered bad prognostic factors in earlier studies.[2],[6],[7],[8],[9],[10],[11] Previous authors have also identified certain histological features such as dense dermal inflammatory infiltrate, tissue eosinophilia and interface dermatitis with or without keratinocyte necrosis as poor prognostic factors in DRESS. Presence of apoptotic keratinocytes in histology was documented to be an indicator of DRESS-induced liver injury.[12],[13] In this setting, we carried out a study among 100 DRESS patients who attended the Dermatology department of our tertiary care institution during a period of 70 months from 1st January 2011 and who required inpatient care to determine the predictors of disease severity in DRESS.
Methods
An analytical cross-sectional study was conducted among 100 drug reactions with eosinophilia and systemic symptoms patients who attended the dermatology outpatient department of the Government Medical College, Kozhikode from 1st January 2011 and who required inpatient care. All cases of definite or probable DRESS diagnosed as per the RegiSCAR DRESS validation scoring system proposed by Kardaun et al. were included in the study after obtaining ethical clearance from the institutional ethics committee and a written informed consent from individual study participants (or guardians in case of children below 18 years).[14],[15]
Exclusion criteria: Drug reactions not satisfying the criteria to be considered as definite or probable drug reaction with eosinophilia and systemic symptoms as per the RegiSCAR validation scoring system were excluded from the study. Patients with DRESS who were not evaluated in the dermatology department and those who did not receive inpatient care in our institution were also excluded from the study.
A preset proforma was used to collect data regarding age, sex and clinical profile of the affected patients. Facial erythema and edema and internal organ involvement, whenever present, were carefully documented in each case. Laboratory investigations including complete hemogram, absolute eosinophil count, liver function (during hospital stay, absolute eosinophil count and liver function tests were repeated once in five days), renal function tests and peripheral smear report were carefully noted.
Disease severity in DRESS was determined (drug reaction with eosinophilia and systemic symptoms severity score) based on the extent of internal organ involvement induced by drug reaction with eosinophilia and systemic symptoms – one point each was awarded for clinical manifestations identified as features of severe DRESS by French Society of Dermatology (transaminases > five times above normal, renal/cardiac involvement, pneumonia and hemophagocytosis). Two points were given for clinical manifestations identified as features of life-threatening DRESS by the French Society of Dermatology (hemophagocytosis with bone marrow failure, encephalitis, severe hepatitis, renal failure and respiratory failure).[5] We defined severe hepatitis as hyperbilirubinemia with elevated liver transaminases.[6]
The data was entered in Microsoft excel and analyzed with SPSS Inc IBM company version 16. The association of age, sex, facial erythema and edema, absolute eosinophil count and atypical lymphocytes in peripheral smear with disease severity in DRESS was assessed using chi-square test. A P value less than 0.05 was considered significant. Analysis of variance with post-hoc test – Bonferroni -- was done wherever possible for multiple comparison and interclass variations. PLUM (polytomous universal model) ordinal regression was used to identify the predictors of disease severity in DRESS. Odds ratio and 95% confidence intervals were assessed.
Results
During the study period of 70 months, we made a diagnosis of probable adverse drug reactions as per the World Health Organization's causality assessment in 272 inpatients.[16] DRESS contributed to 36.8% of the total adverse drug reactions (100/272). Among the 100 patients, 42 were categorized as definite DRESS (42%) and 58 (58%) were probable DRESS as per the RegiSCAR DRESS validation scoring system. Fifty-six were females and the male-to-female ratio was 0.8:1. One female patient had human immunodeficiency virus (HIV) infection and developed DRESS to cotrimoxazole initiated for prophylaxis against Pneumocystis jirovecii pneumonia. There was a single case of connective tissue disease (rheumatoid arthritis) in the study group who developed drug reaction with eosinophilia and systemic symptoms to salazopyrin.
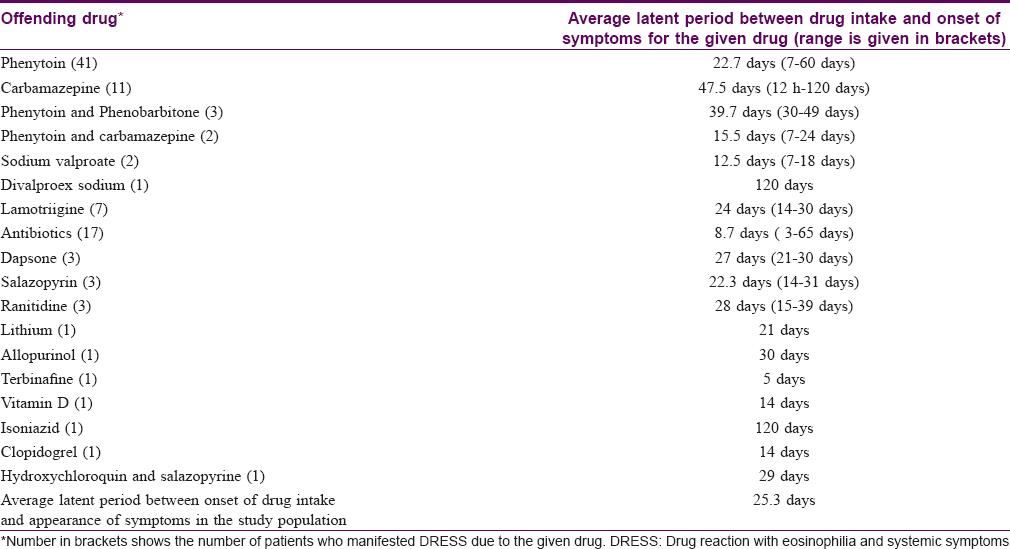
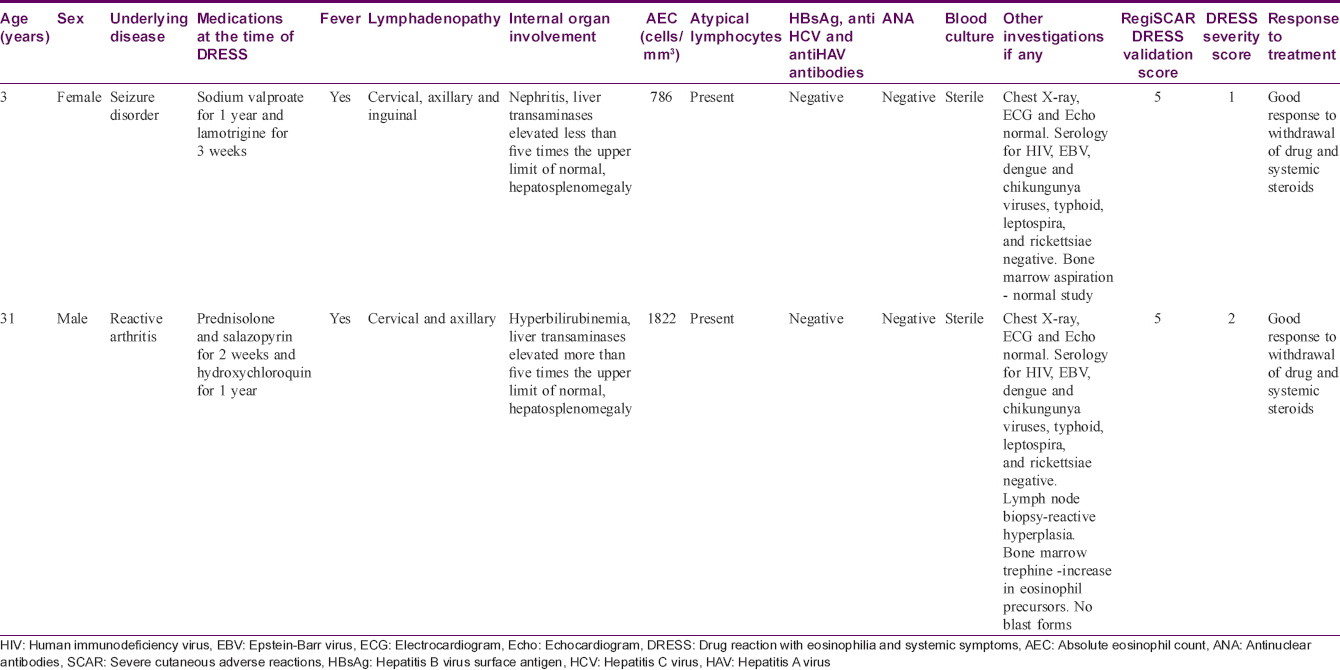
Age of the affected ranged from 2 to 78 years. Mean age of the affected was 38.1 years. Nineteen drugs were identified as producing DRESS [Table - 1]. The most common class of drugs precipitating DRESS was aniconvulsants (in 67 patients, 67%) followed by antibiotics [on 17 occasions (17%) – penicillins 8, cephalosporins, azithromycin and cotrimoxazole 3 each]. The most common drug precipitating DRESS was phenytoin (41 patients, 41%) followed by carbamazepine (11 cases, 11%).
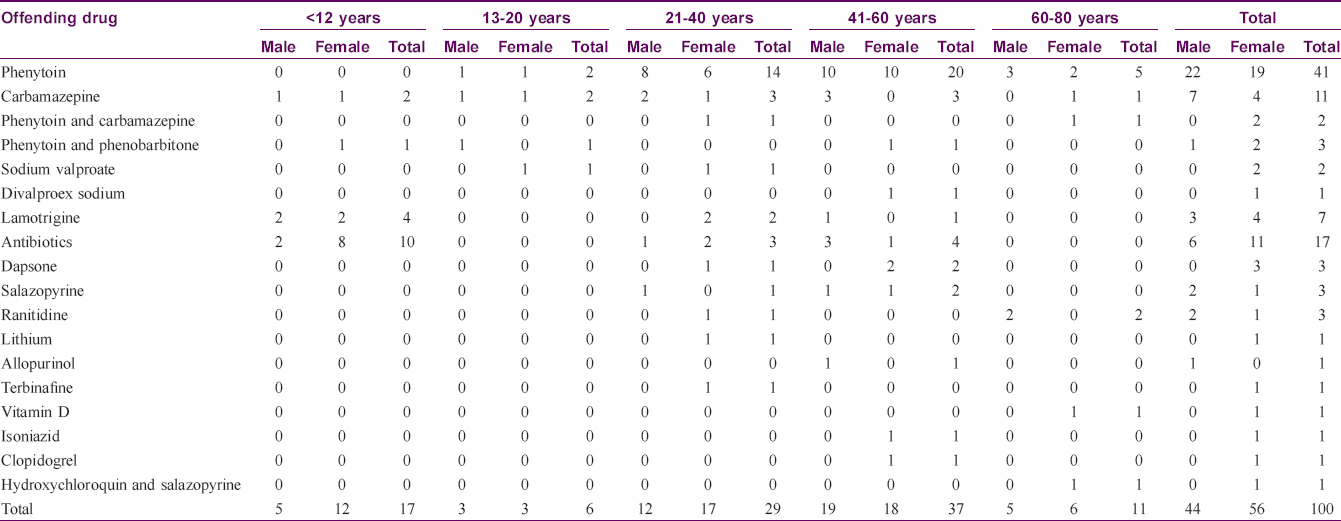
The latent period between the drug intake and the onset of symptoms varied from 72 hours to 120 days with an average of 25.3 days [Table - 2]. In one instance of carbamazepine-induced DRESS, the patient manifested the adverse reaction 72 hours after starting the drug; the patient had taken carbamazepine for seizure disorder without any adverse events which he stopped on his own 3 months later. On restarting the drug for seizure recurrence 2 months later, he manifested features of DRESS within 72 hours. Three of the hundred patients manifested a long latent period of 120 days and the offending drugs were carbamazepine, isoniazid and divalproex sodium. Carbamazepine-induced DRESS patient had hyperbilirubinemia with elevated liver transaminases and had a severity score of two while the other two had no internal organ involvement.
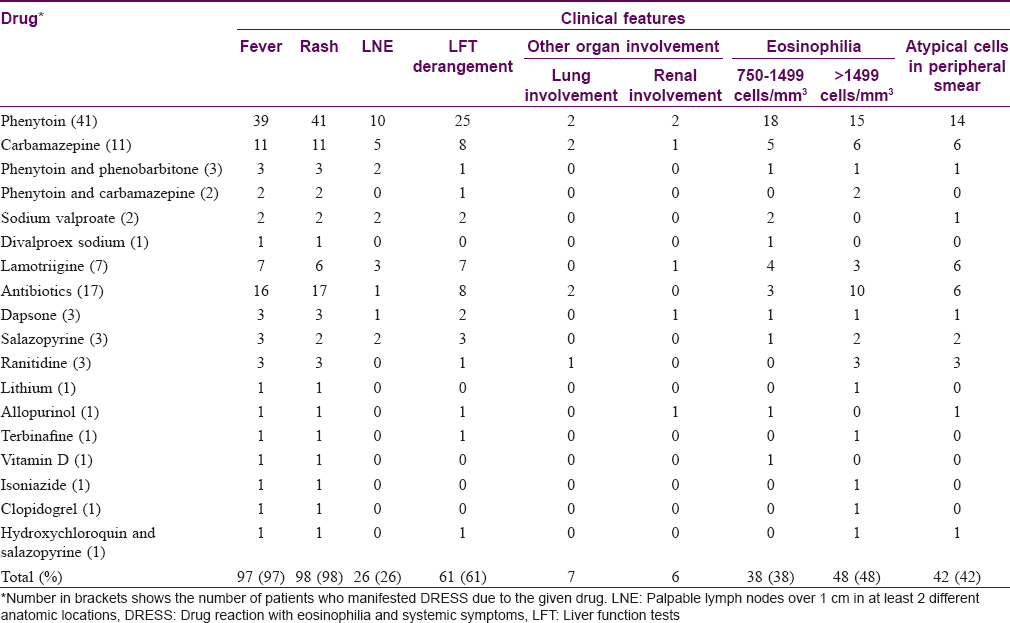
Most common symptoms [Table - 3] observed were rash in 98 patients (98%) [Figure - 1]), fever in 97 patients (97%) and facial edema in 91 patients (91%). Facial erythema and edema were observed in 63 patients (63%). Facial edema or erythema alone was observed in 31 patients (31%). Neither erythema nor edema was documented in 6 cases (6%).
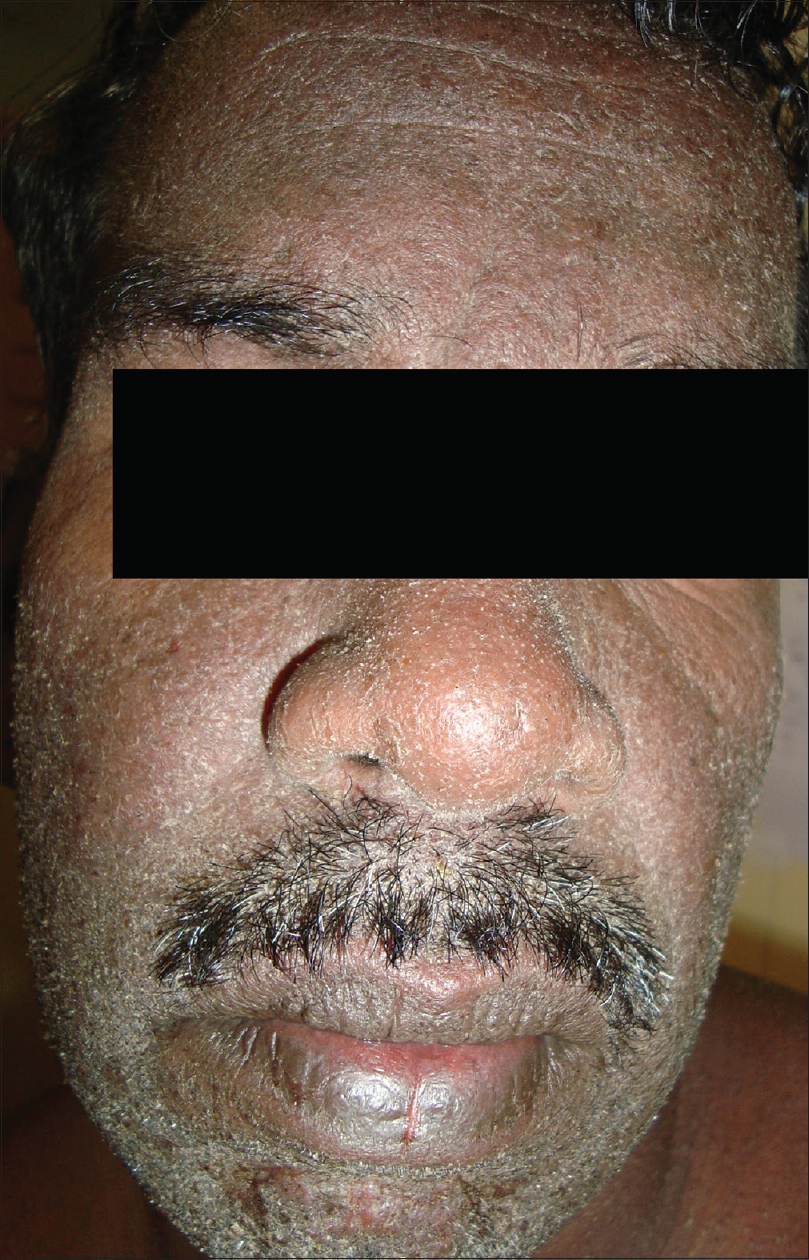 |
| Figure 1: Erythematous scaly rash of drug reaction with eosinophilia and systemic symptoms |
Eighty-five patients (85%) manifested maculopapular rash [Figure - 2]. Other rash types observed were diffuse erythema in 8 cases (8%), erythroderma in 2 cases (2%), infiltrated plaques in 4 patients (4%), purpuric lesions in 2 patients, (2%) [Figure - 3], urticarial lesions in 2 patients (2%) and erythema multiforme in 1 patient (1%). Seven patients (7%) developed pustules scattered over the scalp, face, trunk and extremities. Two patients did not manifest any rash [2%, [Table - 4].[8],[17]
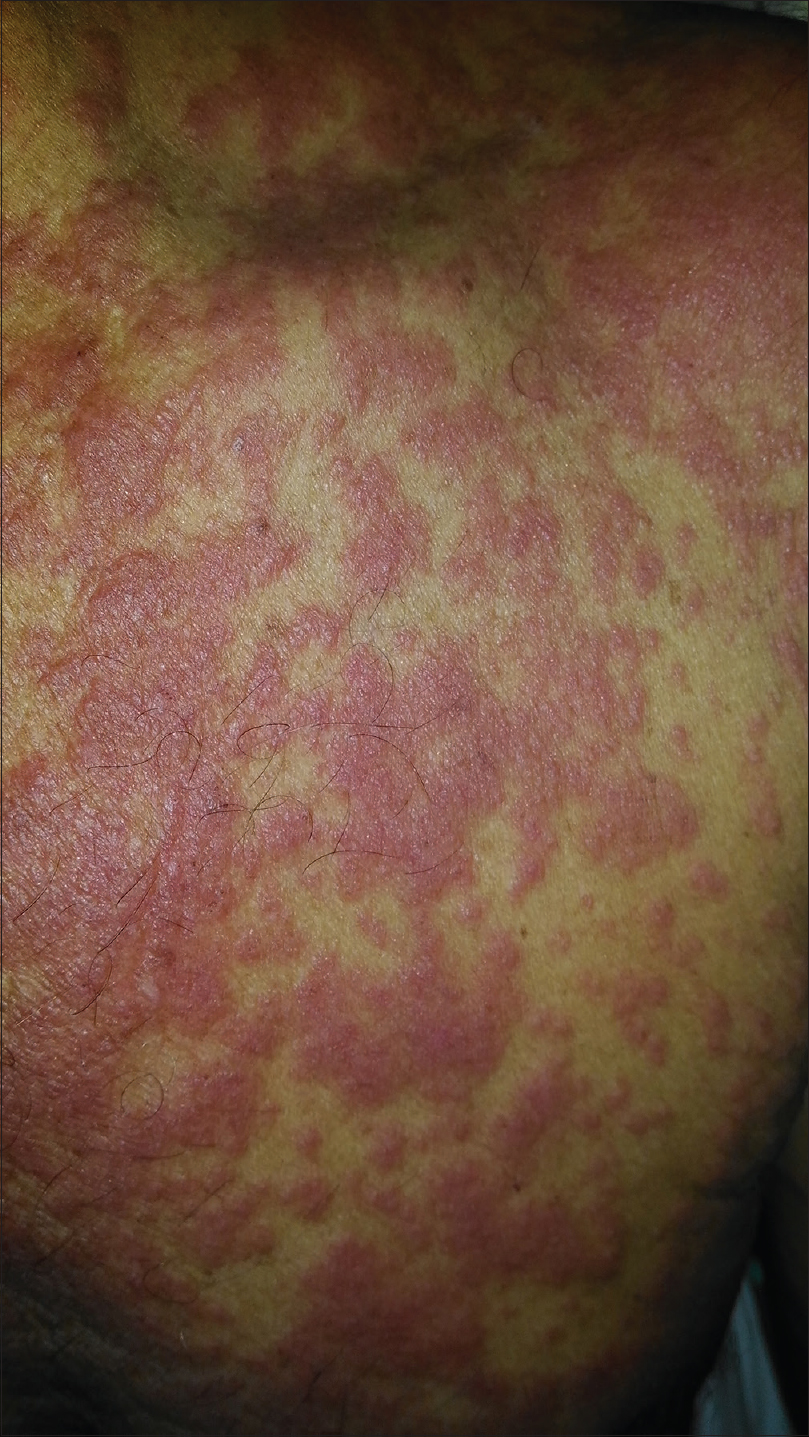 |
| Figure 2: Maculopapular rash in drug reaction with eosinophilia and systemic symptoms |
 |
| Figure 3: Pupuric rash |
Mucosae were spared in nine. Forty-five of the ninety-one patients (49.5%) with mucosal involvement had nonsevere lesions limited to the scaling of lips [Table - 5].

Sixty-four study participants (64%) had involvement of one or more internal organs. Most common systemic involvement noted was elevated liver transaminases in 61 patients (61%). Other organs found affected in the study population were lungs (chest radiography performed for persistent cough and chest pain detected pneumonitis and pleural effusion respectively) and kidneys [Figure - 4]. Renal involvement manifested as elevated blood urea and creatinine in 5 cases and 1 dapsone-induced DRESS patient manifested reduced urine output also. None of the study participants showed tachycardia or tachypnoea.
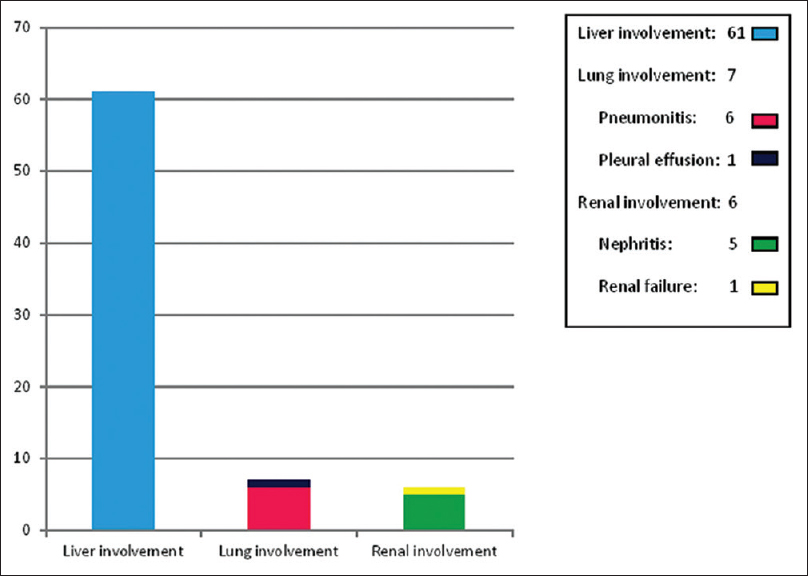 |
| Figure 4: Internal organ involvement in drug reaction with eosinophilia and systemic symptoms |
Absolute eosinophil count was above 1500 cells/mm[3] in 48 patients (48%) and was below 750 cells/mm[3] in fourteen (14%). Peripheral smear showed the presence of atypical lymphocytes in 42 patients (42%).
As per the DRESS severity scoring, 61 patients (61%) had score zero whereas 39 patients (39%) had scores ranging from 1 to 4. The HIV infected patient and the patient suffering from rheumatoid arthritis manifested nonsevere DRESS. [Table - 6] depicts the clinical profile and laboratory data of patients with severity score of one and above and those with DRESS severity score of zero.
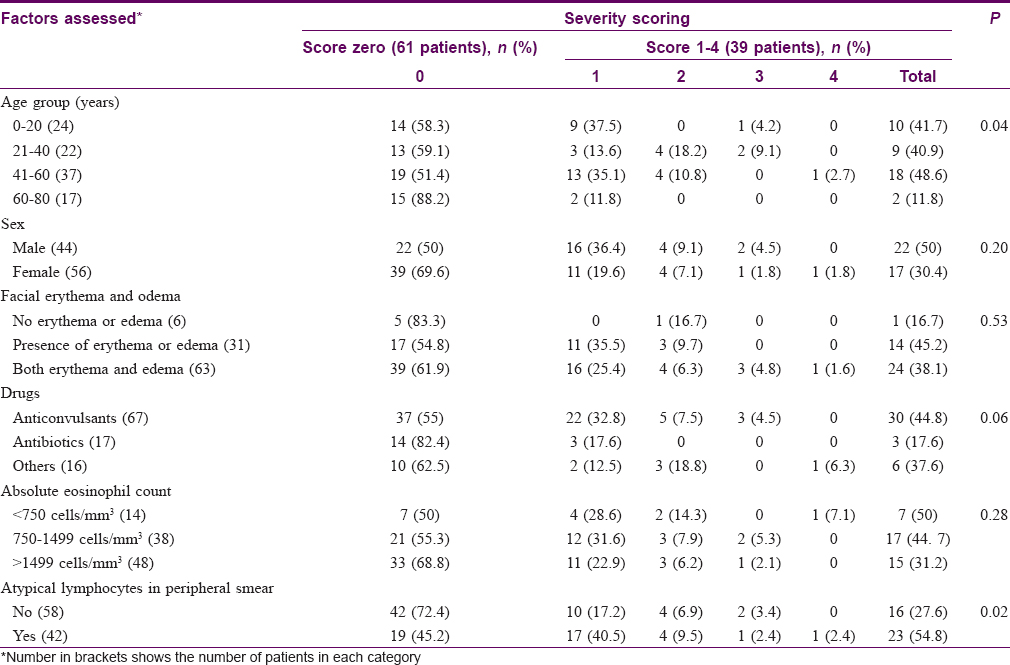
Age was found to have a statistically significant association with disease severity in DRESS (P = 0.04), whereas no statistically significant association was noted for sex, offending drug, facial erythema and edema and absolute eosinophil count. Presence of atypical lymphocytes in peripheral smear showed statistically significant association with severity in DRESS (P = 0.02).
To study the predictors of disease severity in DRESS and for doing away with the effects of confounding factors, analysis with PLUM ordinal regression was done. The model had a significant fit (P = 0.004) and could explain up to 23.1% of the variation in the outcome (Nagelkerke Pseudo R[2]= 0.231).
Age was found to be a significant predictor of disease severity in DRESS. Age groups 0–20, 21–40 and 41–60 years had significantly less odds of severe DRESS compared to age group 61–80 years; [P = 0.03, odds ratio = 7.56 (1.10–51.92); P = 0.02, odds ratio = 8.17 (1.24–53.75); P = 0.02, OR = 7.21 (1.28–40.39), respectively].
Presence of atypical cells in peripheral smear was a significant predictor of disease severity in DRESS. Patients without atypical lymphocytes had 62% less odds of severe DRESS [P = 0.03; odds ratio = 0.38 (0.15–0.92)] as opposed to those with the finding. The results of regression analyses are charted in [Table - 7].

On running analysis of variance and multiple comparison with post hoc tests (Bonferroni alpha), wherever possible, significant interclass variation was found with the presence of atypical lymphocytes (F = 4.27; P = 0.04).
Discussion
Mean age of the participants in our study was similar to the findings of Cacoub et al. but lower than that described in certain other studies.[11],[18] The male-to-female ratio of 0.8:1 noted by us was comparable to the previous reports.[6]
The average latent period between the onset of drug intake and appearance of symptoms documented by us were similar to figures as reported earlier.[11],[18] Though rare, a latent period of 120 days noted in three cases in this study was reported earlier too in other studies, especially in DRESS induced by carbamazepine, allopurinol, lamotrigine and isoniazid.[19],[20],[21],[22] Matta et al. has reported a case of carbamazepine-induced DRESS that appeared 239 days after starting the offending drug and manifested severe hepatic involvement.[19] A similar finding was recorded in the carbamazepine-induced DRESS in our study; however, the other two cases with a prolonged incubation period of 120 days manifested less severe DRESS. We did not come across any previous report of latent period of up to 120 days in divalproex sodium-induced DRESS. The rapid onset of symptoms on second exposure to the same drug noted in one of our patients has been reported previously.[2],[7],[9] Lack of symptoms on first exposure could be attributed to the withdrawal of the drug before development of adequate sensitization for manifestation of symptoms. A shorter latent period between onset of drug intake and appearance of symptoms noted for antibiotic-induced DRESS [Table - 2] could also be due to prior exposure to the same or related drugs.
Literature states that patients receiving prednisolone at a dose of 10 mg or more per day along with another immunosuppressive agent and HIV infected are more likely to manifest DRESS without rash.[23] One of the two patients without rash in our study was receiving prednisolone 10 mg along with hydroxychloroquin (which is an immunomodulator) while he developed rashless DRESS due to salazopyrin [Table - 4].[17] Co-administration of prednisolone and hydroxychloroquin might have played a role in nonmanifestation of rash. The second patient was a 3-year-old child, however, why she developed DRESS without rash remains unclear.[8] Interestingly, the lone HIV positive patient in this study developed rash. She was not on highly active antiretroviral therapy and developed DRESS to cotrimoxazole offered as prophylaxis against Pneumocystis jirovecii pneumonia.
Contrary to the previous observations that underlying HIV infection and collagen vascular diseases place a patient at greater risk for severe drug reaction, both patients suffering from these diseases in the study group had a severity score of zero.[2] However, we are unable to arrive at any definite conclusions because the number was negligible.
Our observation that elderly patients were more likely to develop serious manifestations of DRESS was as described in earlier studies.[10] Contrary to previous data that suggested female sex to be a risk factor for severe manifestations of DRESS, sex was not found to be a predictor for severe DRESS in this study.[10]
In contrast to previous reports, erythema multiforme type rash was not associated with internal organ involvement and severe DRESS in the present study.[12] However, we are unable to arrive at any definite conclusion because only one out of the 100 cases (1%) in our study manifested erythema multiforme lesions.
Unlike previous reports in many studies, facial erythema and edema were not associated with disease severity in DRESS in this study.[7],[10]
Elevated absolute eosinophil count was not identified as a predictor of severe DRESS in the current study. Earlier studies have reported conflicting results on the effect of eosinophilia on the severity of DRESS.[7],[12],[24]
The discrepancies noted between our study and previous studies could be partly attributed to the difference in population characteristics of study participants as none of the previous studies are carried out in this country. Another reason could be the retrospective nature of most previous studies.[10],[11],[12],[18],[19],[20],[21],[22] The few prospective studies that are available are of small sample size.[25]
Presence of atypical lymphocytes in peripheral smear serving as a predictor of disease severity in DRESS in the present study was consistent with our earlier observations.[8],[13]
The main limitations of the present study were our inability to evaluate the role of HLA association and herpes virus reactivation (due to financial constraints) which are recognized as important factors determining the severity of DRESS.[26],[27],[28] The role of histological features as predictors of bad prognosis was not evaluated in this study. Another major drawback was that the current study was neither designed to evaluate mortality caused by DRESS nor was it designed to follow-up the patients to determine the response to treatment and delayed autoimmune complications of DRESS and their predictors.
Conclusions
DRESS is a complex drug reaction which poses a significant diagnostic challenge. The predictors of disease severity in DRESS identified in the current study were the presence of atypical lymphocytes in peripheral smear and age of the affected patients. Further studies with a large sample size in different population groups are needed to confirm or refute our findings which may help us identify the warning signs in DRESS.
Acknowledgment
We are grateful to all the faculty and postgraduates who were part of the department during the study period for their invaluable help in conducting this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Chaiken BH, Goldberg BI, Segal JP. Dilantin sensitivity; report of a case of hepatitis with jaundice, pyrexia and exfoliative dermatitis. N Engl J Med 1950;242:897-8.
[Google Scholar]
|
| 2. |
Criado PR, Avancini J, Santi CG, Medrado AT, Rodrigues CE, de Carvalho JF, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): A complex interaction of drugs, viruses and the immune system. Isr Med Assoc J 2012;14:577-82.
[Google Scholar]
|
| 3. |
Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol 2007;156:1083-4.
[Google Scholar]
|
| 4. |
Gentile I, Talamo M, Borgia G. Is the drug-induced hypersensitivity syndrome (DIHS) due to human herpesvirus 6 infection or to allergy-mediated viral reactivation? Report of a case and literature review. BMC Infect Dis 2010;10:49.
[Google Scholar]
|
| 5. |
Descamps V, Ben Saïd B, Sassolas B, Truchetet F, Avenel-Audran M, Girardin P, et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol 2010;137:703-8.
[Google Scholar]
|
| 6. |
Chen YC, Cho YT, Chang CY, Chu CU. Drug reaction with eosinophilia and systemic symptoms: A drug-induced hypersensitivity syndrome with variable clinical features. J Dermatol Sin 2013;31:196-204.
[Google Scholar]
|
| 7. |
Kumari R, Timshina DK, Thappa DM. Drug hypersensitivity syndrome. Indian J Dermatol Venereol Leprol 2011;77:7-15.
[Google Scholar]
|
| 8. |
Sasidharanpillai S, Riyaz N, Rajan U, Binitha MP, Khader A, Mariyath OK, et al. Drug reaction with eosinophilia and systemic symptoms: Observations from a tertiary care institution. Indian J Dermatol Venereol Leprol 2014;80:221-8.
[Google Scholar]
|
| 9. |
Shiohara T, Kano Y, Takahashi R. Current concepts on the diagnosis and pathogenesis of drug-induced hypersensitivity syndrome. Jpn Med Assoc J 2009;52:347-52.
[Google Scholar]
|
| 10. |
Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: A cause of unpredictable multiorgan failure. Arch Dermatol 2009;145:67-72.
[Google Scholar]
|
| 11. |
Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: A literature review. Am J Med 2011;124:588-97.
[Google Scholar]
|
| 12. |
Walsh S, Diaz-Cano S, Higgins E, Morris-Jones R, Bashir S, Bernal W, et al. Drug reaction with eosinophilia and systemic symptoms: Is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br J Dermatol 2013;168:391-401.
[Google Scholar]
|
| 13. |
Sasidharanpillai S, Govindan A, Riyaz N, Binitha MP, Muhammed K, Khader A, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): A histopathology based analysis. Indian J Dermatol Venereol Leprol 2016;82:28-36.
[Google Scholar]
|
| 14. |
Chen YC, Chang CY, Cho YT, Chiu HC, Chu CY. Reply to: “Using a diagnostic score when reporting the long-term sequelae of the drug reaction with eosinophilia and systemic symptoms”. J Am Acad Dermatol 2013;69:1060-2.
[Google Scholar]
|
| 15. |
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br J Dermatol 2007;156:609-11.
[Google Scholar]
|
| 16. |
The Use of the WHO-UMC System for Standardized Case Causality Assessment. Uppsala: The Uppsala Monitoring Centre; 2005. Available from: http://www.who-umc.org/Graphics/24734.pdf. [Last accessed on 2014 Jan 04].
[Google Scholar]
|
| 17. |
Sasidharanpillai S, Binitha MP, Manikath N, Janardhanan AK. Drug reaction with eosinophilia and systemic symptoms without skin rash. Indian J Pharmacol 2015;47:687-9.
[Google Scholar]
|
| 18. |
Ang CC, Wang YS, Yoosuff EL, Tay YK. Retrospective analysis of drug-induced hypersensitivity syndrome: A study of 27 patients. J Am Acad Dermatol 2010;63:219-27.
[Google Scholar]
|
| 19. |
Matta JM, Flores SM, Cherit JD. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An Bras Dermatol 2017;92:30-3.
[Google Scholar]
|
| 20. |
Chan JC, Chan HH, Yeung CK. Drug reaction with eosinophilia and systemic symptoms (DRESS). Hong Kong J Dermatol Venereol 2012;20:163-70.
[Google Scholar]
|
| 21. |
Nam YH, Park MR, Nam HJ, Lee SK, Kim KH, Roh MS, et al. Drug reaction with eosinophilia and systemic symptoms syndrome is not uncommon and shows better clinical outcome than generally recognised. Allergol Immunopathol (Madr) 2015;43:19-24.
[Google Scholar]
|
| 22. |
Wang L, Mei XL. Drug reaction with eosinophilia and systemic symptoms: Retrospective analysis of 104 cases over one decade. Chin Med J (Engl) 2017;130:943-9.
[Google Scholar]
|
| 23. |
Ben m'rad M, Leclerc-Mercier S, Blanche P, Franck N, Rozenberg F, Fulla Y, et al. Drug-induced hypersensitivity syndrome: Clinical and biologic disease patterns in 24 patients. Medicine (Baltimore) 2009;88:131-40.
[Google Scholar]
|
| 24. |
Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: A retrospective study of 60 cases. Arch Dermatol 2010;146:1373-9.
[Google Scholar]
|
| 25. |
Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, et al. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: A study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol 2008;22:1044-9.
[Google Scholar]
|
| 26. |
Descamps V, Bouscarat F, Laglenne S, Aslangul E, Veber B, Descamps D, et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br J Dermatol 1997;137:605-8.
[Google Scholar]
|
| 27. |
Descamps V, Mahe E, Houhou N, Abramowitz L, Rozenberg F, Ranger-Rogez S, et al. Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br J Dermatol 2003;148:1032-4.
[Google Scholar]
|
| 28. |
Kano Y, Hiraharas K, Sakuma K, Shiohara T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol 2006;155:301-6.
[Google Scholar]
|
Fulltext Views
4,574
PDF downloads
1,992





