Translate this page into:
Prevalence and risk factors of onychomycosis in primary school children living in rural and urban areas in Central Anatolia of Turkey
2 Department of Public Health, Erciyes University, Kayseri, Turkey
3 Department of Public Health, Gulhane Military Medical Faculty, Ankara, Turkey
4 Department of Infectious Diseases, Turkish Armed Forces Health Command, Ankara, Turkey
5 Department of Microbiology, Erciyes University, Kayseri, Turkey
Correspondence Address:
Mustafa Gulgun
Department of Pediatrics, Gulhane Military Medical Faculty, 06010, Ankara
Turkey
| How to cite this article: Gulgun M, Balci E, Karaoglu A, Kesik V, Babacan O, Fidanci MK, Turker T, Tok D, Koc N. Prevalence and risk factors of onychomycosis in primary school children living in rural and urban areas in Central Anatolia of Turkey. Indian J Dermatol Venereol Leprol 2013;79:777-782 |
Abstract
Background: Onychomycosis is a world-wide public health concern in children, requiring epidemiological data for different regions for control and prevention. Aim: The aim of this study was to evaluate the predominant pathogens and risk factors for onychomycosis in school children living in Kayseri, Turkey. Methods: This study included 8122 school children, aged 5-16 years, living in the rural and urban areas around Kayseri. Onychomycosis was clinically classified as distal and lateral subungual (DLSO), proximal subungual, superficial white, endonyx and totally dystrophic onychomycosis. Nail samples from children with clinically diagnosed onychomycosis were collected, examined by direct microscopy and inoculated for culture study. The demographic features and possible risk factors were recorded and assessed by logistic regression models. Results: We clinically diagnosed onychomycosis in 152 out of 8,122 (0.18%) school children. DLSO was the most frequent clinical diagnosis (120/152, 78.9%). Culture-positive onychomycosis was detected in 27/152 (17.7%) children. The prevalence of culture-positive onychomycosis was determined as 0.33%. All culture-positive samples were only from toenails. The onychomycosis causative agents were dermatophytes in 17/27 cases (62.9%), including Trichophyton rubrum 12 (44.4%), Trichophyton mentagrophytes 1 (3.7%), Trichophyton tonsurans 1 (3.7%) and Trichophyton spp. 3 (11.1%) and yeasts in 10/27 cases (37.1%), including Candida glabrata 4 (14.8%), Candida parapsilosis 1 (3.7%), Trichosporon 2 (7.4%) and Rhodotorula 3 (11.1%). Age, father's occupation, number of siblings and rooms were statistically associated with the frequency of onychomycosis. Conclusions: Although to be prevalence of onychomycosis in school children in central Anatolia of Turkey seems very low degree, pediatric onychomycosis is a growing public health concern all over the world. Children having more siblings or unemployed fathers and children living in small house as well as older children should be examined carefully for onychomycosis.Introduction
Onychomycosis can be caused by dermatophytes, yeasts and non-dermatophyte molds that are transmitted through infected moist floor areas and less often transmitted via direct personal contact. [1] In Europe, North America and Turkey, the most common etiologic agents of onychomycosis are dermatophytes, in children and adults, whereas yeasts are the most common in Saudi Arabia and Pakistan in the general population. [2],[3],[4],[5]
Non-dermatophyte molds are accepted as uncommon or secondary pathogens in onychomycosis in already damaged nails by trauma, ischemia or disease, especially dermatophyte infection and frequently seen in elderly, immunosuppression, poor peripheral circulation or temperate climates. [6],[7],[8] The prevalence of onychomycosis is low among children compared with adults due to reduced exposure to infected environments (communal showers, public changing rooms and saprophytic fungi), faster linear nail growth, less cumulative trauma due to smaller and thinner nail surface and lower prevalence of tinea pedis. [8],[9]
Epidemiologic surveys depicted the prevalence of onychomycosis in children <16 years of age ranging from 0.2% to 2.6%. [10] The prevalence of onychomycosis varies depending on age, sex, regional differences, cultural habits, migration, seasonal conditions, immune status of the host, living and hygienic conditions. [11] It is therefore essential to obtain epidemiological data for different regions to enable strategic planning for control and prevention. No data are available on the prevalence and common etiologic agents of onychomycosis in children in our region. [6],[7],[11],[12]
The aim of this study was to estimate the prevalence of onychomycosis in school children living in rural and urban areas of central Anatolia of Turkey and to determine the risk factors for infection.
Methods
This cross-sectional epidemiological study was conducted in school children in Kayseri, a city with a total of 139,422 school children in central Anatolia, Turkey. The climate in Kayseri is cold, snowy and wet in winter while dry and hot in summer. The lowest and highest temperature in Kayseri are −5.2°C and +25.7°C (mean 7.73°C). Mean humidity is 69.3%. This study was approved by the Erciyes University Ethics Committee. A total of 8,122 children, aged 5-16 years, from randomly selected 24 primary schools located in Kayseri were selected by cluster sampling method and examined for onychomycosis at the first visit. Clinical examination of fingernails and toenails was performed by a pediatrician and a medical physician specialized on public health at the same time. Inclusion criteria were school children aged 5-16 year-old studying in Kayseri. Exclusion criteria were <5 or >16 years old.
Onychomycosis was clinically classified as distal and lateral subungual (DLSO), proximal subungual (PSO), superficial white (SWO), endonyx and totally dystrophic (TDO) onychomycosis. If there was onycholysis and subungual hyperkeratosis, thickening or distortion of the nail plate, it was diagnosed as DLSO. It was considered as PSO in the evidence of subungual hyperkeratosis, transverse leukonychia, proximal onycholysis or destruction of the proximal nail plate. The diagnosis of SWO was defined as homogenously white nail, diffusely opaque with variable pigmentation, flexible and friable. Nails with diffuse milky-white discoloration, with normal thickness and normal plate surface, in the absence of nail bed hyperkeratosis or onycholysis were diagnosed as endonyx. If there was total destruction of the entire nail plate including whole thickness of the plate, the nail bed and matrix and if dystrophic and thick nail crumbled and disappeared leaving a thickened abnormal nail bed retaining keratotic nail debris, this clinical pattern was diagnosed as TDO. Paronychia was determined if there was painful swelling and erythema of the proximal and lateral nail folds. [13] When onychomycosis was clinically diagnosed, samples from clinically suspected nails after cleaning with 70% alcohol were collected by scraping or shaving from the distal portion of the nail, the proximal nail bed, the undersurface of the nail plate, the friable area of leukonykia, hyperkeratotic nail bed, opaque white area and proximal, distal and lateral nail edge with a disposable scalpel or curette. Onycholytic nail plate was removed before sampling. Outermost debris was discarded. Child′s feet with abnormal onychomycosis-suspected areas were also examined for desquamation and/or scaling, plantar fissures, discoloration, and groove. When tinea pedis was suspected, samples from the feet skin, interdigital surfaces, toe web or the surrounding skin were collected. After samples were examined with 15% potassium hydroxide (KOH) solution by direct microscopy, the samples were cultured according to literature. [14] Isolated yeast that did not form a germ tube was identified by the growth properties in corn-meal agar and by using ID 32 C (Bio-Merieuxi, Marcy I′Etoile and France). Dermatophytes when isolated were accepted as the causative agent and a patient of onychomycosis was diagnosed when a positive culture was detected for a dermatophyte.
C. albicans was regarded as the primary pathogen on repeated isolation along with a direct microscopy outcome demonstrating yeast pseudomycelia. Non-C. albicans spp. were admitted as the primary pathogen with two or more isolation as long as there was yeast pseudomycelia in direct microscopic examination and no other concomitant pathogen. Candida spp. was taken into account as the secondary pathogen if they were isolated with dermatophyte or non-dermatophyte pathogenic mold with microscopy revealing budding yeast cells.
The following details were recorded for each child: Age, sex, school grade, number of siblings, parents′ educational and occupational status, family income, frequency of having baths (per week) and sock changing, animal husbandry, school settlement, number of rooms and types of shoes.
Statistical analysis was conducted using the package SPSS 15.0 (Chicago, IL). Categorical variables were defined as the number and percentage (%) and analyzed using the Chi-square test. P values less than 0.05 were accepted as statistically significant. The dependent variable in multivariate models was the presence of culture-positive dermatophytic infection. Odds ratios with 95% confidence intervals (CI 95%) were calculated from the coefficients.
Results
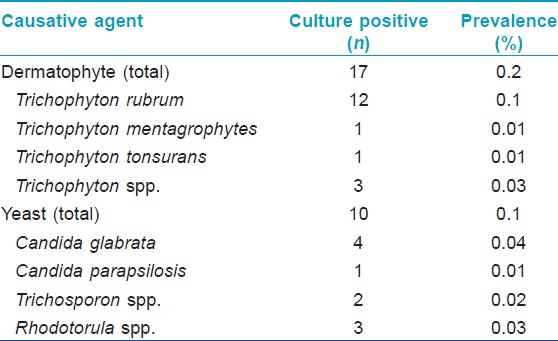
A total of 8122 children, including 4,032 (49.6%) boys and 4,090 (50.4%) girls, with a mean age of 10.61 ± 2.41 (range 5-16) were examined. Nine fingernail and 143 toenail scrapings were taken from children clinically having onychomycosis. The mean age of these 152 children (73 boys and 79 girls) was 11.9 ± 2.2 years (5-16 years). In fingernails, 6/9 (66.6%) and 3/9 (33.3%) patients were diagnosed as DLSO and PSO respectively. In toenails, DLSO was the most frequent clinical diagnosis (114, 73.6%), followed by PSO (16, 10.5%), SWO (4, 2.6%), TDO (6, 3.9%), endonyx (3, 1.9%) [Table - 1]. Paronychia was determined in 14 patients with DLSO. Discoloration from yellow-brown to black was the prominent clinical sign in all patients. Hyphae or spores were seen in 65/152 (42.8%) scraping materials by direct microscopy and cultures were positive in 27/65 (41.5%). Onychomycosis were detected in 27/152 (17.8%) patients by isolation. The prevalence of culture-positive onychomycosis was 0.33% (27/8122). There were no fungal KOH-positive or culture-positive cases from fingernails. Toenails were affected in all of the fungal culture-positive cases. Dermatophytes (62.9%) occurred more commonly than yeasts (37.1%) in culture-positive onychomycosis cases. The most common agents in the DLSO were Trichophyton rubrum, Candida spp. and less often, Rhodotorula [Table - 2]. Three out of 27 (11.1%) fungal culture-positive cases also had infection of the foot skin caused by the same fungal species, including Trichosporon spp., Candida glabrata and Rhodotorula spp. The culture positive cases were 23/114 in DLSO, 2/16 in PSO, 1/4 in SWO and 1/6 in TDO [Table - 1]. Culture results in cases with paronychia were positive in 4/14 due to C. glabrata (3), Candida parapsilosis (1) and these cases also had culture-positive onychomycosis with the same causative agents.
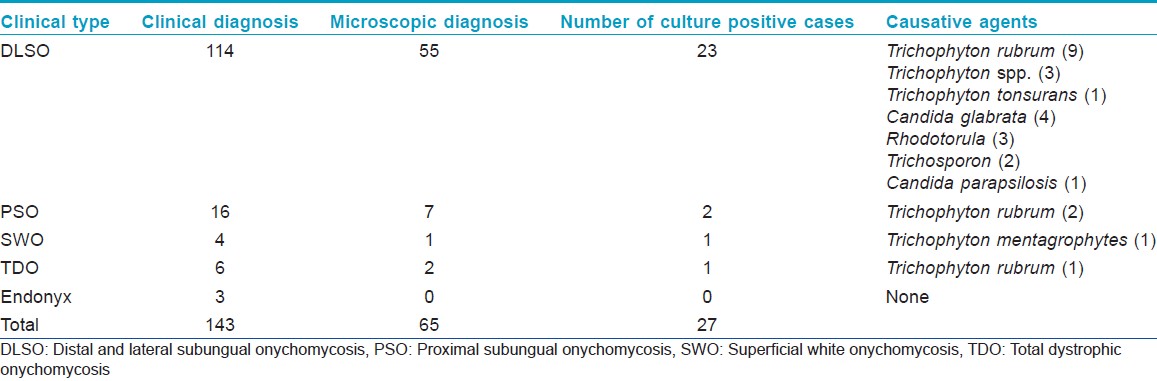
Children aged ≥10 years were more likely to present with onychomycosis (0.55%), compared with the <10 years old group (0.11%) (P = 0.001). Onychomycosis in children having ≥3 siblings (0.45%) was higher than for those having 1-2 siblings (0.04%) (P = 0.002). Onychomycosis in children having unemployed fathers (0.67%) was higher than for those whose fathers were employed (0.21%) (P = 0.003). Onychomycosis in children living in the rural area (0.49%) was found to be more prevalent than those in children living in the urban area (0.25%) (P < 0.001). Onychomycosis in children living in a house with <3 rooms (0.73%) was higher than for those living in a house with ≥3 rooms (0.27%) (P = 0.014). Onychomycosis in children wearing rubber shoes (0.64%) was higher than for those wearing non-rubber shoes (0.10%) (P < 0.001). There was no statistically significant association between onychomycosis and sex, parent′s education, mother′s occupation, animal husbandry, family income, frequency of having a bath or sock changing.
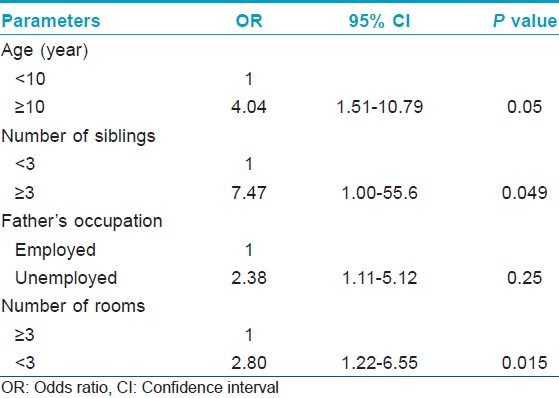
Age, father′s occupation, number of siblings and rooms were found as potential risk factors for onychomycosis in multivariate logistic model [Table - 3].
Discussion
Onychomycosis is considered among the most common nail diseases in childhood along with eczema, psoriasis, lichen planus, onychodystrophy, alopecia areata and genodermatoses. [6],[14] Dystrophic nails must alert the clinician to the possibility of onychomycosis and should be differentiated from other acquired and congenital conditions. [15] To our knowledge, this is the first comprehensive study of onychomycosis performed in children living in rural and urban areas in central Anatolia, Turkey. In our study, the overall prevalence of onychomycosis was 0.33% and T. rubrum was the most prominent isolate as dermatophytes and C. glabrata as yeast. Our study also showed that age, parent′s occupational status, number of siblings and rooms were important factors for the frequency of onychomycosis.
The prevalence of onychomycosis is low in children. Studies from North-America and Europe reported the prevalence of onychomycosis as 0.44% and 0.6% in children less than 18 years of age. [16],[17] The prevalence of onychomycosis in primary school children of Israel and Spain were reported as 0.87% and 0.15%, respectively, [17],[18] Two separate studies conducted in Turkey demonstrated the prevalence of onychomycosis as 0.1% and 0.08%, respectively. [6],[19] The prevalence of onychomycosis was very low in our study, similar to previous literature.
In the 1950s, epidemiological studies performed in Turkey reported that the most prevalent causative isolate was Epidermophyton floccosum. [20] However, T. rubrum was detected as the main pathogen for onychomycosis in this study, which was similar to that in Turkey, India, Western United States, Northern and Southern Europe and was different from that in Africa, supporting the finding that the flora of dermatophytes has recently changed. [5],[9],[19],[20],[21],[22],[23] A significant increase in the prevalence of T. rubrum over the last decades may perhaps be due to the greater availability of fungi in the environment and in adults after prolongation of life accompanied by various diseases facilitating the transfer of fungi to children. [11],[18],[23]
Yeasts can cause onychomycosis and mixed infections with dermatophytes are also possible. [24] Reports of onychomycosis caused by Candida spp. in children are less than those caused by dermatophytes. Although C. albicans and C. parapsilosis are two frequently isolated species, we found that the dominant causative agent for onychomycosis from yeast was C. glabrata, followed by Rhodotorula and Trichosporon.[1] A study reported that Trichosporon was the most common causative agent for onychomycosis in children living in the West of Turkey. [6] Diagnosing children with Rhodotorula and Trichosporon infections, suggests that these emerging yeasts should also be considered an opportunistic primary causative agent of onychomycosis. [25],[26] However, isolation of different causative agents may be a result of variations in geographic and climatic conditions as well as different relevant methodological aspects, such as sample size, source of participants, type of evaluation and definition of onychomycosis. [27]
This study supported that onychomycosis affected mainly toenails in children compared to fingernails. This could be the result of more traumas and using occlusive footwear or tight fitting shoes, which favors occurrence of onychomycosis. We could not show fingernail onychomycosis by microscopic or culture studies. It might be associated with technical issues or inspection of fingernails during hygienic inspections by teachers, causing awareness of any fingernail diseases earlier. Nevertheless, fingernail onychomycosis seems to be very rare in children living in Kayseri. [28],[29],[30]
DLSO type could be seen in children and adults and was the most common clinical pattern in our study, in agreement with previous reports. [10],[21] It is known that SWO is very rare in children. [13] Similarly, we detected only one case of SWO in our study. Total dystrophic onychomycosis has also been reported very rarely in children probably due to the faster growth rate of nails compared to adults. [1] We detected only one case with TDO caused by T. rubrum. Onychomycosis was associated with tinea pedis in three patients (11.1%); although, this association was found as high as 47% in previous studies. [1]
Onychomycosis prevalence is higher in adults than in children and tends to become more common over the years. [10] Our study showed that onychomycosis was 4-fold (CI: 95% 1.51-10.79) more common in children aged ≥10 years than those aged <10 years, in agreement with other studies. [9],[10],[11],[18] This can be related to increasing usage of occlusive shoes and more sporting activities resulting in more traumas with increasing age.
Pιrez-Gonzαlez et al. [31] reported male gender as a risk factor for onychomycosis in Spanish children, similar to reports from Turkey. [6],[19] The reason for this difference has not been understood very well. Sex may influence susceptibility to some of the fungal infections like onychomycosis. [31] In our study, sex did not seem to be a risk factor for onychomycosis, in contrast to some previous reports.
Although, it was reported that wearing occlusive footwear and excessive sweating of the feet were important factors in tinea pedis, which might be a facilitating factor for onychomycosis, wearing sweating rubber shoes did not seem to be a risk factor for onychomycosis in our study, even though most of the children with onychomycosis proven to be positive by culture were wearing rubber shoes. [32]
It is difficult to compare our results with the findings of other studies because of few investigations assessing the risk factors for onychomycosis. In our study, the prevalence in children living in a house with ≤2 rooms was 2.8-fold (CI: 95% 1.22-6.55) higher compared to those living in a house with ≥3 rooms. A study from Turkey reported more frequent dermathophytic infections in children living in a dormitory, compatible with our results. [19] The prevalence in children having ≥3 siblings was 7.4-fold (CI: 95% 1.0-55.6) more than those having ≤2 siblings, similar to that in literature. [33] The higher prevalence in children living in a small house and having more siblings may be attributed to crowded environment causing close proximity to each other. Having unemployed father increased the prevalence rate by 2.38-fold (CI: 95% 1.22-6.55). All factors mentioned above might cause poor living standards resulting in increase in the prevalence.
In conclusion, although prevalence of onychomycosis in school children in central Anatolia seems very low, onychomycosis is a growing public health concern all over the world. Children having more siblings or unemployed fathers and children living in small house as well as older children should be examined carefully for onychomycosis. Physicians should consider this infection in the differential diagnosis of diseases affecting nails and provide valuable epidemiological data on future efforts for the prevention and treatment of onychomycosis.
| 1. |
Lange M, Roszkiewicz J, Szczerkowska-Dobosz A, Jasiel-Walikowska E, Bykowska B. Onychomycosis is no longer a rare finding in children. Mycoses 2006;49:55-9.
[Google Scholar]
|
| 2. |
Bokhari MA, Hussain I, Jahangir M, Haroon TS, Aman S, Khurshid K. Onychomycosis in Lahore, Pakistan. Int J Dermatol 1999;38:591-5.
[Google Scholar]
|
| 3. |
Abanmi A, Bakheshwain S, El Khizzi N, Zouman AR, Hantirah S, Al Harthi F, et al. Characteristics of superficial fungal infections in the Riyadh region of Saudi Arabia. Int J Dermatol 2008;47:229-35.
[Google Scholar]
|
| 4. |
Svejgaard EL, Nilsson J. Onychomycosis in Denmark: Prevalence of fungal nail infection in general practice. Mycoses 2004;47:131-5.
[Google Scholar]
|
| 5. |
Akcaglar S, Ener B, Toker SC, Ediz B, Tunali S, Tore O. A comparative study of dermatophyte infections in Bursa, Turkey. Med Mycol 2011;49:602-7.
[Google Scholar]
|
| 6. |
Gunduz T, Metin DY, Sacar T, Hilmioglu S, Baydur H, Inci R, et al. Onychomycosis in primary school children: Association with socioeconomic conditions. Mycoses 2006;49:431-3.
[Google Scholar]
|
| 7. |
Yeniºehirli G, Bulut Y, Sezer E, Günday E. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol 2009;48:956-9.
[Google Scholar]
|
| 8. |
Thomas J, Jacobson GA, Narkowicz CK, Peterson GM, Burnet H, Sharpe C. Toenail onychomycosis: An important global disease burden. J Clin Pharm Ther 2010;35:497-519.
[Google Scholar]
|
| 9. |
Young LS, Arbuckle HA, Morelli JG. Onychomycosis in the denver pediatrics population, a retrospective study. Pediatr Dermatol 2012. [In Press].
[Google Scholar]
|
| 10. |
Rodríguez-Pazos L, Pereiro-Ferreirós MM, Pereiro M Jr, Toribio J. Onychomycosis observed in children over a 20-year period. Mycoses 2011;54:450-3.
[Google Scholar]
|
| 11. |
Koksal F, Er E, Samasti M. Causative agents of superficial mycoses in Istanbul, Turkey: Retrospective study. Mycopathologia 2009;168:117-23.
[Google Scholar]
|
| 12. |
Kiraz N, Metintas S, Oz Y, Koc F, Koku Aksu EA, Kalyoncu C, et al. The prevalence of tinea pedis and tinea manuum in adults in rural areas in Turkey. Int J Environ Health Res 2010;20:379-86.
[Google Scholar]
|
| 13. |
Singal A, Khanna D. Onychomycosis: Diagnosis and management. Indian J Dermatol Venereol Leprol 2011;77:659-72.
[Google Scholar]
|
| 14. |
Andrews MD, Burns M. Common tinea infections in children. Am Fam Physician 2008;77:1415-20.
[Google Scholar]
|
| 15. |
Ploysangam T, Lucky AW. Childhood white superficial onychomycosis caused by Trichophyton rubrum: Report of seven cases and review of the literature. J Am Acad Dermatol 1997;36:29-32.
[Google Scholar]
|
| 16. |
Gupta AK, Sibbald RG, Lynde CW, Hull PR, Prussick R, Shear NH, et al. Onychomycosis in children: Prevalence and treatment strategies. J Am Acad Dermatol 1997;36:395-402.
[Google Scholar]
|
| 17. |
Piérard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: A pan-European survey. Dermatology 2001;202:220-4.
[Google Scholar]
|
| 18. |
Leibovici V, Evron R, Dunchin M, Westerman M, Ingber A. A population-based study of toenail onychomycosis in Israeli children. Pediatr Dermatol 2009;26:95-7.
[Google Scholar]
|
| 19. |
Metintas S, Kiraz N, Arslantas D, Akgun Y, Kalyoncu C, Kiremitçi A, et al. Frequency and risk factors of dermatophytosis in students living in rural areas in Eskiºehir, Turkey. Mycopathologia 2004;157:379-82.
[Google Scholar]
|
| 20. |
Ilkit M. Onychomycosis in Adana, Turkey: A 5-year study. Int J Dermatol 2005;44:851-4.
[Google Scholar]
|
| 21. |
Sarma S, Capoor MR, Deb M, Ramesh V, Aggarwal P. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol 2008;47:584-7.
[Google Scholar]
|
| 22. |
Popoola TO, Ojo DA, Alabi RO. Prevalence of dermatophytosis in junior secondary schoolchildren in Ogun State, Nigeria. Mycoses 2006;49:499-503.
[Google Scholar]
|
| 23. |
Tsoumani M, Jelastopulu E, Bartzavali C, Vamvakopoulou S, Dimitracopoulos G, Anastassiou ED, et al. Changes of dermatophytoses in southwestern Greece: An 18-year survey. Mycopathologia 2011;172:63-7.
[Google Scholar]
|
| 24. |
Moreno G, Arenas R. Other fungi causing onychomycosis. Clin Dermatol 2010;28:160-3.
[Google Scholar]
|
| 25. |
da Cunha MM, dos Santos LP, Dornelas-Ribeiro M, Vermelho AB, Rozental S. Identification, antifungal susceptibility and scanning electron microscopy of a keratinolytic strain of Rhodotorula mucilaginosa: A primary causative agent of onychomycosis. FEMS Immunol Med Microbiol 2009;55:396-403.
[Google Scholar]
|
| 26. |
Sageerabanoo S, Malini A, Oudeacoumar P, Udayashankar C. Onychomycosis due to Trichosporon mucoides. Indian J Dermatol Venereol Leprol 2011;77:76-7.
[Google Scholar]
|
| 27. |
Perea S, Ramos MJ, Garau M, Gonzalez A, Noriega AR, del Palacio A. Prevalence and risk factors of tinea unguium and tinea pedis in the general population in Spain. J Clin Microbiol 2000;38:3226-30.
[Google Scholar]
|
| 28. |
Sigurgeirsson B, Kristinsson KG, Jonasson PS. Onychomycosis in Icelandic children. J Eur Acad Dermatol Venereol 2006;20:796-9.
[Google Scholar]
|
| 29. |
Godoy-Martinez P, Nunes FG, Tomimori-Yamashita J, Urrutia M, Zaror L, Silva V, et al. Onychomycosis in São Paulo, Brazil. Mycopathologia 2009;168:111-6.
[Google Scholar]
|
| 30. |
Romano C, Papini M, Ghilardi A, Gianni C. Onychomycosis in children: A survey of 46 cases. Mycoses 2005;48:430-7.
[Google Scholar]
|
| 31. |
Pérez-González M, Torres-Rodríguez JM, Martínez-Roig A, Segura S, Griera G, Triviño L, et al. Prevalence of tinea pedis, tinea unguium of toenails and tinea capitis in school children from Barcelona. Rev Iberoam Micol 2009;26:228-32.
[Google Scholar]
|
| 32. |
Triviño-Duran L, Torres-Rodriguez JM, Martinez-Roig A, Cortina C, Belver V, Perez-Gonzalez M, et al. Prevalence of tinea capitis and tinea pedis in Barcelona school children. Pediatr Infect Dis J 2005;24:137-41.
[Google Scholar]
|
| 33. |
Inanir I, Sahin MT, Gündüz K, Dinç G, Türel A, Arisoy A, et al. Case Report. Tinea pedis and onychomycosis in primary school children in Turkey. Mycoses 2002;45:198-201.
[Google Scholar]
|
Fulltext Views
4,622
PDF downloads
3,035





