Translate this page into:
Primary carcinoma of accessory breast presenting as axillary ulcer
Corresponding author: Dr. Chengjun Cui, Department of Dermatology, Wuxi Branch of Zhongda Hospital, Southeast University, Wuxi City, Jiangsu Province, Wuxi, China. 0082002003@163.com.
-
Received: ,
Accepted: ,
How to cite this article: Chen C, Cui C. Primary carcinoma of accessory breast presenting as axillary ulcer. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_301_2024
Dear Editor,
A 50-year-old man presented to the dermatology clinic with a one-year history of recurrent discharging ulcer in the left axilla. The lesion initially appeared as asymptomatic erythematous plaques for which he received antibiotics and infrared therapy at a community health center. The skin lesions improved temporarily but recurred 2 weeks later. Due to financial constraints, the patient did not seek further treatment. Over the next 6 months, the lesions progressively enlarged and subsequently developed ulceration. The lesion edges were sharp and irregular, with visible proliferative granulation tissue on the surface [Figure 1]. Patient denied a history of trauma, exposure to chemical substances or radiation. An incisional biopsy from the ulcer revealed a moderately differentiated adenocarcinoma. The tumour cells were arranged in solid nests or cord-like patterns, with focal areas showing glandular arrangement and infiltrative growth. Prominent nucleoli and nuclear mitotic figures were observed [Figure 2a]. Immunohistochemistry showed positivity for estrogen receptor (ER) [Figure 2b], progesterone receptor (PR) [Figure 2c], cytokeratin (CK), CK7, GATA3 and Mammaglobin, and negativity for CDX2, GCDFP-15, NapsinA, TTF-1 and Villin. The expression of HER2 was 2+ by immunohistochemistry [Figure 2d], and Ki67 showed 30% positivity. The magnetic resonance imaging (MRI) revealed no primary lesion in the ipsilateral breast tail and bilateral mammary glands, but multiple enlarged lymph nodes were found in the left axilla. A diagnosis of primary accessory breast carcinoma was made. The patient underwent extended resection of the left axillary tumour with a 2 cm horizontal margin and left axillary lymph node dissection, followed by 3 courses of EC chemotherapy (epirubicin at 90 mg/m2, and cyclophosphamide at 600 mg/m2). At 3-months follow-up, the patient remained tumour-free.
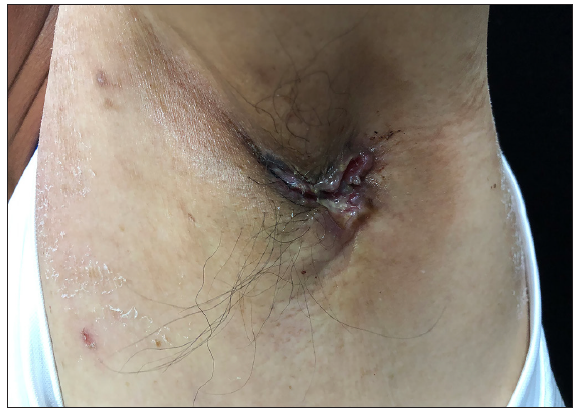
- Discharging ulcer of the left axilla with sharp and irregular edges.
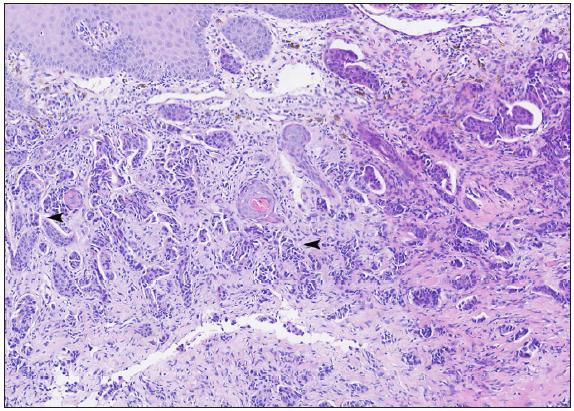
- Histopathology demonstrates invasive ductal carcinoma (black arrowheads) in the dermis (Haematoxylin and Eosin, 100x).
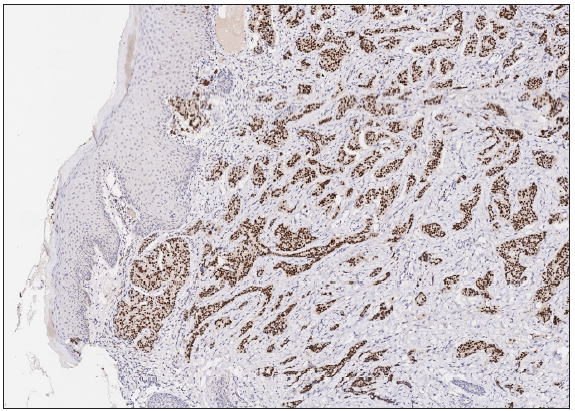
- Tumour cells were positive for Estrogen Receptor (ER) (Immunohistochemistry, 100x).
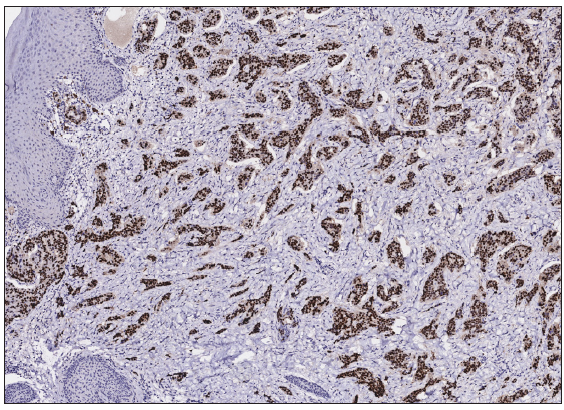
- Tumour cells were positive for Progesterone Receptor (PR) (Immunohistochemistry, 100x).
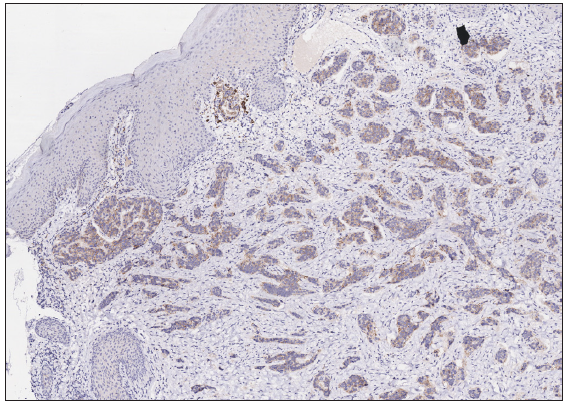
- The expression of Human Epidermal Growth Factor Receptor 2 (HER2) was 2+ (Immunohistochemistry, 100x).
Accessory breast cancer is extremely rare, accounting for 0.3%–0.6% of all breast cancers, especially in male patients. Accessory breasts result from the incomplete regression of normal breast tissue and occur at a rate of 1%–6%. The male-to-female ratio for the occurrence of accessory breasts is approximately 1:5.1 It is believed that Asians have a higher incidence of accessory breast carcinoma compared to Caucasians.2 For lumps presenting along the breast line (from axilla to groin), accessory mammary tumours should be considered. Clinically, the lesion initially appears as erythematous (red) papules or nodules and gradually develops into firm masses with unclear boundaries. As the condition advances, tumour can protrude through the skin and lead to ulceration. Imaging, including ultrasound (USG), computed tomography (CT), MRI and positron emission tomography (PET)-CT, provide objective visualisation of tumour morphology and precise tumour localisation, which are significantly important in the differential diagnosis of breast cancer. Pathology remains the diagnostic gold standard.3 The predominant histopathological features observed in accessory breast cancer are invasive ductal carcinoma and poorly differentiated adenocarcinoma.
Presentation of an axillary ulcer associated with accessory breast carcinoma should first be distinguished from axillary lymphadenitis and lymph node tuberculosis. Axillary lymphadenitis, commonly caused by Staphylococcus aureus and haemolytic Streptococcus, presents with erythema, oedema, heat and pain, which can be mitigated by prompt antibiotic and anti-inflammatory therapy. Axillary lymph node tuberculosis, caused by Mycobacterium tuberculosis, begins with firm, painless lymph nodes that can develop into perilymphadenitis and result in necrosis, abscesses and ulceration, with occasional systemic symptoms including fever, night sweats and anorexia. Clinically, it is also necessary to differentiate from axillary Crohn’s disease and hidradenitis suppurativa. Axillary Crohn’s disease is often accompanied by abdominal pain and diarrhoea, making endoscopic examination particularly important. Hidradenitis suppurativa is generally bilateral with comedones, inflammatory papules, nodules, cysts, abscesses, sinus tracts, and fistulas. For a persistent non-healing ulcer in the axilla with indurated margins, continuous progression, and no positive findings on pathological examination, it is necessary to consider neoplastic conditions, including accessory mammary carcinoma. In addition, further differentiation by histopathological and imaging examinations is required to distinguish it from conditions such as metastatic breast cancer, apocrine gland carcinoma and squamous cell carcinoma. In our case, MRI showed no occult lesions or other primary malignant tumours, particularly in the mammary gland. Apocrine gland carcinoma is characterised by large tumour cells that exhibit positivity on Periodic Acid-Schiff staining. In this case, the tumour exhibited a growth pattern consistent with breast cancer, presenting as invasive ductal carcinoma without decapitated secretions. With the positive expression of breast-derived immunohistochemical markers, we diagnosed accessory breast carcinoma.
There are currently no specific guidelines for the treatment of accessory breast cancer. The main treatment is radical resection with axillary lymph node dissection, followed by adjuvant radiotherapy, chemotherapy, and endocrine therapy.4 Recently, there have been reports on the use of trastuzumab monotherapy for the treatment of metastatic male breast cancer.5 Considering that this disease can easily be overlooked, dermatologists should be aware of this rare tumour.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Male accessory breast cancer on the abdominal wall: A case report and literature review. Onco Targets Ther. 2018;11:6625-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Breast cancer occurring in the chest wall: Rare presentation of ectopic milk line breast cancer. J Clin Oncol. 2014;32:e35-e36.
- [CrossRef] [PubMed] [Google Scholar]
- Accessory breast cancer: A case report and literature review of ultrasonographic findings. Australas J Dermatol. 2023;64:e384-e385.
- [CrossRef] [PubMed] [Google Scholar]
- Axillary masses as clinical manifestations of male sweat gland carcinoma associated with extramammary Paget’s disease and accessory breast carcinoma: Two cases report and literature review. World J Surg Oncol. 2022;20:109.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Male accessory breast cancer successfully treated with single-agent trastuzumab: A case report. Am J Mens Health. 2023;17:15579883231171010.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





