Translate this page into:
Primary cutaneous anaplastic large cell lymphoma in a child simulating primary cutaneous Hodgkin's disease
Correspondence Address:
Vikram K Mahajan
Department of Dermatology, Venereology and Leprosy, Dr. R. P. Govt. Medical College, (Tanda) - 176 001, Himachal Pradesh
India
| How to cite this article: Mahajan VK, Jindal R. Primary cutaneous anaplastic large cell lymphoma in a child simulating primary cutaneous Hodgkin's disease. Indian J Dermatol Venereol Leprol 2016;82:98-101 |
Sir,
The spectrum of CD30+ lymphoproliferative disorders includes a variety of T-cell lymphomas with lymphomatoid papulosis and anaplastic large-cell lymphoma at two ends of the spectrum. Anaplastic CD30+ large-cell lymphoma is the second most common, constituting 9% of all cutaneous lymphomas. It is a large T-cell lymphoma consisting of cells with an anaplastic, pleomorphic or immunoblastic cytomorphology and is also characterized by expression of the CD30 (Ki-1) antigen by >75% of the tumor cells.[1] It primarily involves lymph nodes and also has frequent cutaneous and extra-cutaneous involvement (nodal, systemic). It affects both children and adults and carries a poor prognosis. Primary cutaneous anaplastic large-cell lymphoma is currently considered an infrequent but distinct entity.
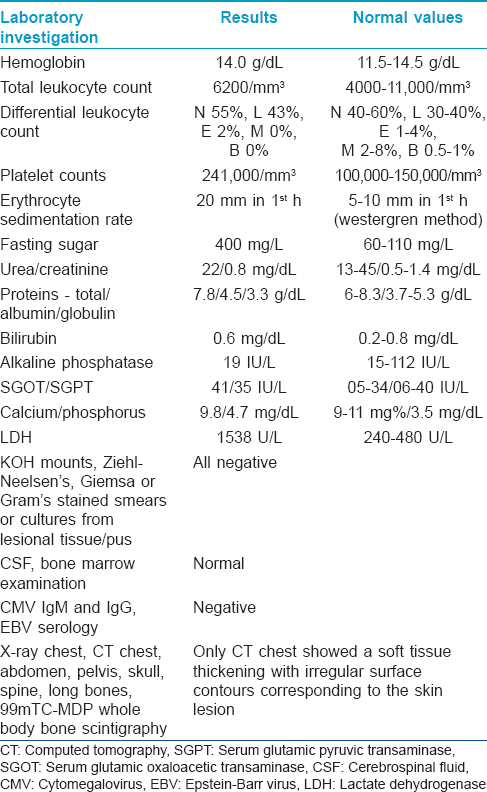
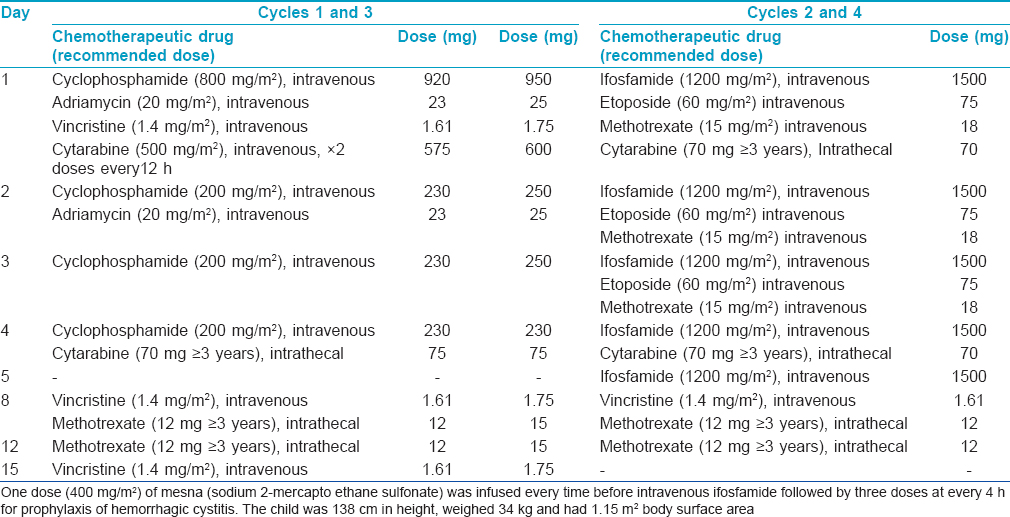
A 11-year-old boy presented with two progressively enlarging nodules over the left breast of 1 week duration with signs of impending ulceration. He had no fever, night sweats or weight loss (B symptoms). The lesions ulcerated the following day, discharging a profuse amount of blood mixed with pus leaving a large ulcer over the upper medial quadrant of the left breast which spared the nipple-areola complex [Figure - 1]. The ulcer was freely mobile and had well defined, irregular, rolled out margins with hyperpigmented borders and a fleshy floor. An enlarged lymph node was found in the left axilla which was non tender, mobile, firm and had normal overlying skin. Systemic examination and investigations were carried out with the provisional diagnoses of pyoderma gangrenosum, subcutaneous mycosis and cutaneous tuberculosis, and revealed no abnormalities [Table - 1]. A fine needle aspiration cytology from the axillary lymph node demonstrated very cellular smears comprising of highly pleomorphic large cells with abundant deeply basophilic cytoplasm and large, single to multiple pleomorphic nuclei with fine chromatin and single to multiple nucleoli. These features were suggestive of anaplastic large cell lymphoma (pleomorphic histiocytoid lymphoma) or polymorphous subtype of immunoblastic lymphoma. Many of these cells had multilobulated and eccentrically placed nuclei. A large number of mitotic figures, some with a bizarre appearance were also noted. Histologic examination of the skin biopsy specimen from the ulcer also showed features suggestive of primary cutaneous anaplastic large-cell lymphoma [Figure - 2],[Figure - 3],[Figure - 4]. Immunohistochemical studies revealed CD30+ large cells [Figure - 5] that stained negative for CD20, CD8, CD3, CD4 LCA, pan CK and anaplastic lymphoma kinase 1 (ALK1) favoring the diagnosis of primary cutaneous anaplastic large-cell lymphoma (Stage 1). Studies for chromosomal abnormality or epithelial membrane antigen (EMA) expression were not performed as they were unavailable/unaffordable. Further investigations revealed no evidence of any systemic disease [Table - 1]. He recovered completely after treatment with the pediatric oncology group protocol 842 chemotherapy without significant adverse events [Table - 2]. There was no recurrence in the more than 5 years follow-up period.
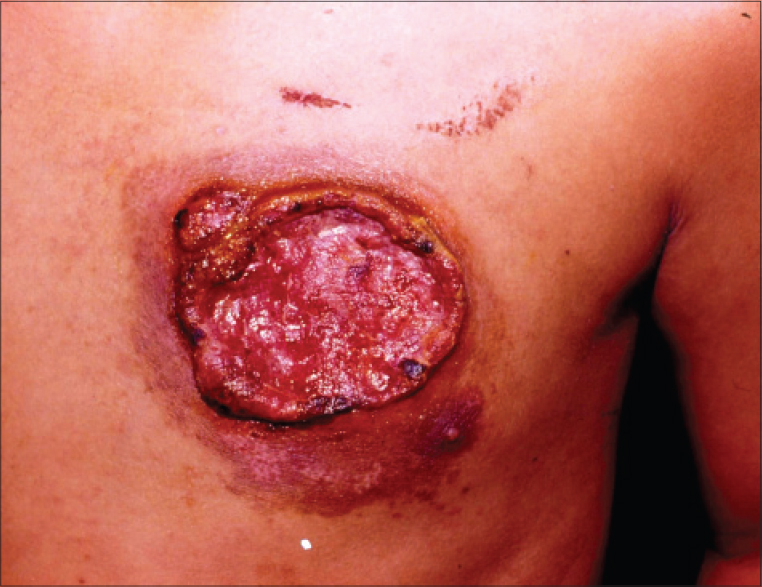 |
| Figure 1: A large ulcer of 6 cm × 4 cm size with well defined, irregular, rolled out borders and fl eshy fl oor over left breast. Furthermore, note another coalescing small ulcerated plaque with hyperpigmented periphery, sparing the areola/nipple. The ill-defi ned swelling in the left axilla is due to the enlarged lymph node |
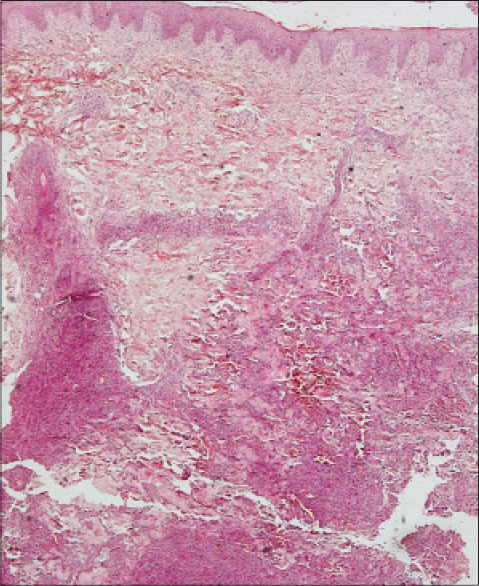 |
| Figure 2: Focally eroded epidermis, no defi nite epidermotropism, mixed infl ammatory cells in the upper dermis and cohesive sheets of diffuse lymphoid infi ltrate in deep dermis and subcutis (H and E, ×100) |
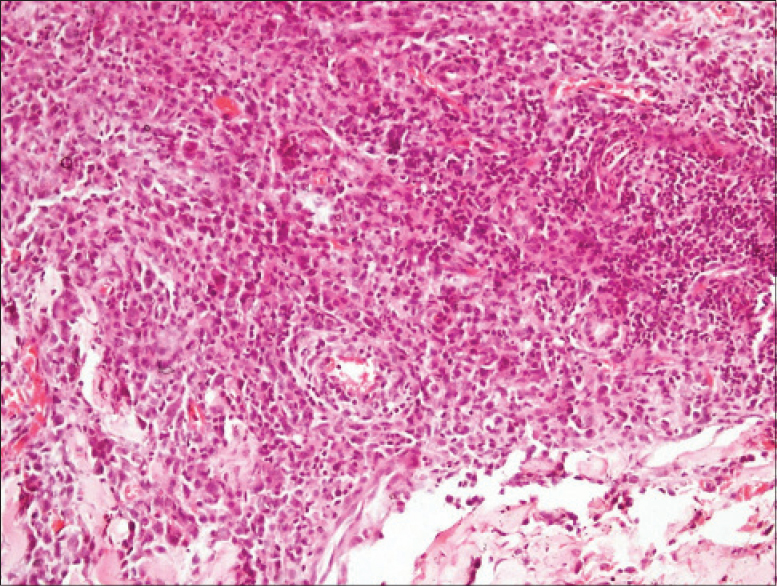 |
| Figure 3: Many large atypical cells, some with an indented nucleus and a moderate amount of cytoplasm: the “hallmark cells,” marked pleomorphism and mitotic fi gures. Large hyperchromatic cerebriform cells or eosinophilic infi ltrate is conspicuously absent (H and E, ×400) |
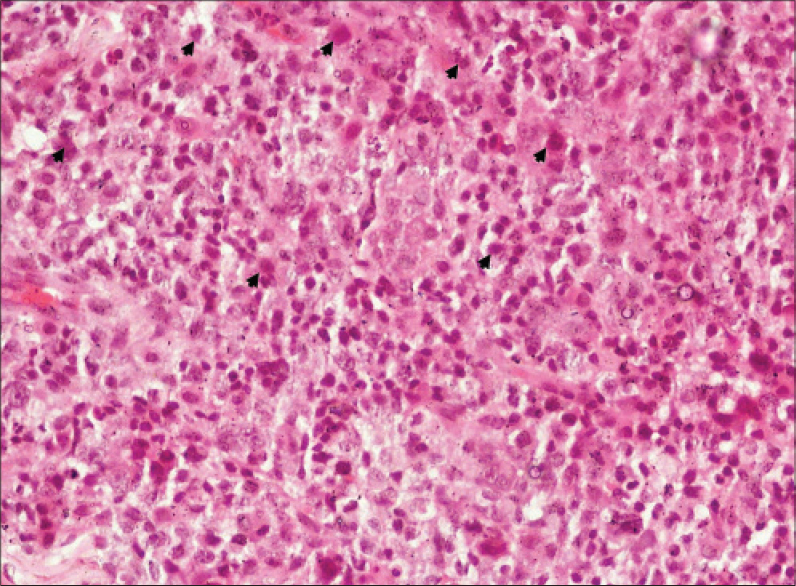 |
| Figure 4: The “hallmark cells”, pleomorphism and mitotic fi gures (arrowheads) (H and E, ×400) |
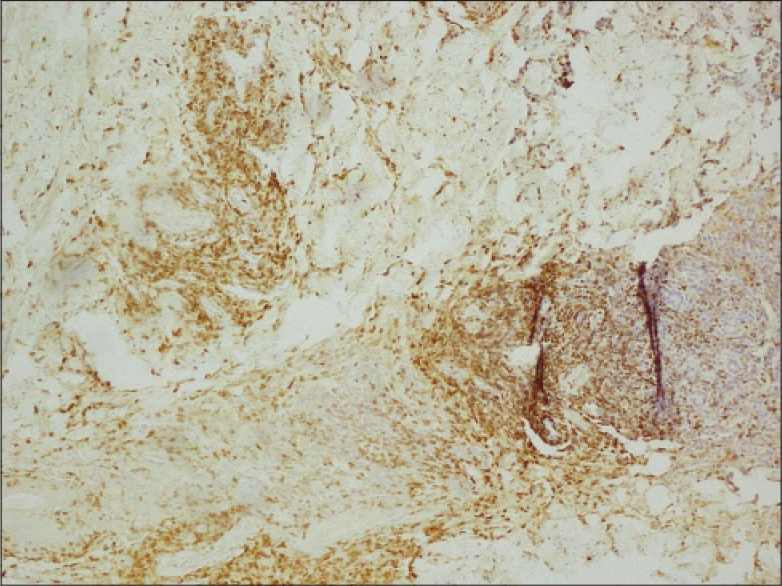 |
| Figure 5: Majority of the infi ltrating cells in the upper dermis and cohesive sheets of diffuse lymphoid infi ltrate in deep dermis and subcutis are CD30+ (×100) |
Cutaneous CD30+ anaplastic large-cell lymphoma is a lymphoma of B-, T-, or null-cell origin with a heterogeneous clinicopathologic and cytological spectrum. It was once considered a high-grade non-Hodgkin's lymphoma and accounts for nearly 3% of adult and 10% of childhood cases. Primary cutaneous anaplastic large-cell lymphoma without nodal or visceral involvement at presentation is uncommon before 20 years of age (median age of onset 60 years), affects males more often (male: female: 2–3:1) and has an indolent clinical course.[2] Infrequent extra-cutaneous dissemination, spontaneous remissions (in 25% cases) or low recurrence rates after therapy and favorable prognosis are the other features.[3] It also lacks interchromosomal t(2;5) translocation (a unique p23:q35 chromosomal abnormality) and expression of EMA or ALK1 protein by the tumor cells.[3],[4],[5] Clinically, it presents as rapidly growing, asymptomatic red-brown tumoral nodule(s) or plaque(s) with or without ulceration simulating infectious/inflammatory dermatoses or may mimic melanoma, metastatic carcinoma or sarcoma. However, disseminated or grouped multiple lesions, 25% of them with regional lymph node involvement, occur in about 20% of cases.[6] Conversely, the involvement of liver, spleen and/or systemic/regional lymph nodes is a consistent feature of primary systemic or nodal anaplastic large-cell lymphoma. Cutaneous anaplastic large-cell lymphoma may follow the classic systemic form, start de-novo or arise from anaplastic transformation of nearly half of other lymphomas such as mycosis fungoides (CD8+, CD20+ with loss of CD2, CD3, CD5), Sézary syndrome (CD3+, CD4+, CD7−, CD8−) or Hodgkin's lymphoma. Immunosuppression (HIV infection, post-organ transplant) and drug therapy (cyclosporine, glatiramer acetate) are the other reported causes. Morphologically, the features of anaplastic large-cell lymphoma are similar to those of high-grade non-Hodgkin's lymphoma. However, all clinical and pathologic subtypes of anaplastic large-cell lymphoma will consistently exhibit strong CD30 (Ki-1) expression on immunophenotyping.[7] Non-classic subtypes of anaplastic large-cell lymphoma including small cell type, lympho-histiocytic subtype and Hodgkin-like will additionally have EMA expressed by most tumor cells.[7],[8] Histologically, diffuse non-epidermotropic infiltrates of cohesive sheets of large CD30+ anaplastic tumor cells or “hallmark cells” of classic morphology is pathognomic. These “hallmark cells” have round, oval or irregularly-shaped embryonic nuclei, prominent multiple nucleoli and abundant cytoplasm. However, a pleomorphic or immunoblastic appearance may occur in 20–25% of cases.[2] Large hyperchromatic cerebriform cells are characteristic of secondary anaplastic large-cell lymphoma and the presence of eosinophilic infiltrate is an indicator of lymph node involvement.[9] Lymphomatoid papulosis and cutaneous Hodgkin's lymphoma are important histologic differentials. The initial de novo skin lesion in our patient confined to the skin around the nipple area without eroding the actual nipple tissue is considered more characteristic of cutaneous Hodgkin's lymphoma.[10] Since primary cutaneous Hodgkin's lymphoma without systemic disease is uncommon, some workers even consider such previously reported cases as anaplastic large-cell lymphoma or lymphomatoid papulosis in view of the lack of specificity associated with Reed–Sternberg cells.[11],[12] Prognostically, primary cutaneous anaplastic large-cell lymphoma has low malignant potential despite the morphologic features of non-Hodgkin's lymphoma and the high-grade histological appearance. Nevertheless, in view of the poorly understood prognostic factors, rapid disease progression and recurrences, early and effective treatment may result in favorable long-term prognosis. Moreover, systemic anaplastic large-cell lymphoma may initially present as isolated cutaneous lesions progressing to extra-cutaneous disease with regional nodal involvement, similar to our case, and eventually with systemic disease from retrograde spread.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985;66:848-58.
[Google Scholar]
|
| 2. |
Newlove T, Loyd A, Patel R, Jelinek J, Latkowski JA. Primary cutaneous anaplastic large-cell lymphoma. Dermatol Online J 2010;16:2.
[Google Scholar]
|
| 3. |
Kempf W, Pfaltz K, Vermeer MH, Cozzio A, Ortiz-Romero PL, Bagot M, et al. EORTC, ISCL, and USCLC consensus CD-30 positive lymphoproliferative disorders: Lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphomas. Blood 2011;118:4024-35.
[Google Scholar]
|
| 4. |
DeCoteau JF, Butmarc JR, Kinney MC, Kadin ME. The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30 lymphoproliferative disorders: Comparison with anaplastic large-cell lymphoma of nodal origin. Blood 1996;87:3437-41.
[Google Scholar]
|
| 5. |
ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Pulford K, Willemze R, Falini B, et al. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J Clin Pathol 2000;53:445-50.
[Google Scholar]
|
| 6. |
Kumaran MS, Jithendriya M, Nagaraj P, Tirumalae R, Jayaseelan E. Anaplastic lymphoma kinase-positive primary cutaneous anaplastic large cell lymphoma – Is it a new variant? Indian J Dermatol Venereol Leprol 2012;78:354-7.
[Google Scholar]
|
| 7. |
Cerroni L, Gatter K, Kerl H, editors. Primary cutaneous CD30+ lymphoproliferative disorderes. In: Skin Lymphoma: The Illustrated Guide. 3rd ed. Oxford: Wiley-Blackwell; 2009. p. 66-86.
[Google Scholar]
|
| 8. |
Massone C, El-Shabrawi-Caelen L, Kerl H, Cerroni L. The morphologic spectrum of primary cutaneous anaplastic large T-cell lymphoma: A histopathologic study on 66 biopsy specimens from 47 patients with report of rare variants. J Cutan Pathol 2008;35:46-53.
[Google Scholar]
|
| 9. |
Beljaards RC, Kaudewitz P, Berti E, Gianotti R, Neumann C, Rosso R, et al. Primary cutaneous CD30-positive large cell lymphoma: Definition of a new type of cutaneous lymphoma with a favorable prognosis. A European Multicenter Study of 47 patients. Cancer 1993;71:2097-104.
[Google Scholar]
|
| 10. |
MacKie RM, editor. Cutaneous lymphomas, pseudolymphomas and histiocytosis X: Hodgkin's disease. In: Skin Cancer: An Illustrated Guide to the Aetiology, Clinical Features, Pathology and Management of Benign and Malignant Cutaneous Tumours. 2nd ed. London: Martin Dunitz Ltd., 1996. p. 231-3.
[Google Scholar]
|
| 11. |
Mukesh M, Shuttleworth D, Murray P. Primary cutaneous Hodgkin's lymphoma. Clin Exp Dermatol 2009;34:e673-5.
[Google Scholar]
|
| 12. |
Cerroni L, Gatter K, Kerl H, editors. Cutaneous manifestations of Hodgkin's lymphoma. In: Skin Lymphoma: The Illustrated Guide. 3rd ed. Oxford: Wiley-Blackwell; 2009. p. 225-8.
[Google Scholar]
|
Fulltext Views
3,576
PDF downloads
1,922





