Translate this page into:
Primary cutaneous CD4 positive small/medium T cell lymphoma
2 Department of Histopathology, Dr Lal PathLabs, New Delhi, India
Correspondence Address:
Gaurav Jain
95/H34/3, Rohini, New Delhi - 110 085
India
| How to cite this article: Jain G, Aiyer HM. Primary cutaneous CD4 positive small/medium T cell lymphoma. Indian J Dermatol Venereol Leprol 2018;84:186-188 |
Sir,
A 79-year-old male presented with complaints of ulcerated lesion on the left forehead. He gave a history of a tiny nodule appearing 6 months back, which was gradually increasing in size since then. There was no history of fever, itching, weight loss, night sweats, pain in the abdomen, or any other similar swelling anywhere else in the body. On examination, there was a single reddish ulcerated nodular lesion measuring 1.5 × 1 cm on the left forehead. No evidence of any scaly plaque/patch was noted. No palpable cervical lymph nodes were present. Systemic examination was unremarkable.
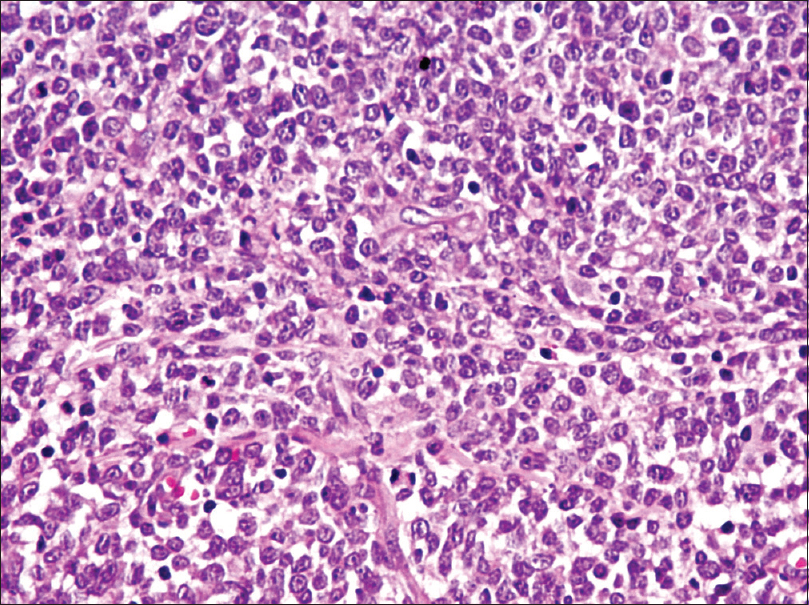
Excisional skin biopsy was performed with a provisional diagnosis of granulomatous lesion and was sent for histopathological examination. Clinical differentials that were considered were cutaneous tuberculosis or fungal granulomatous lesion; however, microsections showed nodular infiltrate of atypical cells in the dermis. Infiltrate was involving the entire dermal thickness in an interstitial and periadnexal pattern with nodular extension into the subcutis. It had a polymorphous composition with predominance of small to medium-sized pleomorphic cells with mild to moderate cytological atypia. The atypical cells exhibited elongated, hyperchromatic nuclei with indistinct nucleoli, and few showed irregular nuclear membranes. Few histiocytes and rare scattered large lymphoid cells were present (<30%) [Figure - 1] and [Figure - 2]. With the possibility of an atypical lymphoid proliferative lesion, immunohistochemical evaluation of the atypical cells was performed. These atypical cells were CD4 + ve, CD8 –ve, CD3 + ve, CD5 + ve, CD30 –ve, CD20 –ve, epithelial membrane antigen –ve, terminal deoxyribonucleic nucleotide transferase –ve, and epstein barr virus encoded RNA –ve [Figure - 3] and [Figure - 4]. Proliferative index (Ki67) was 15%. The atypical cell population was composed of T lymphoid cells as evident by CD3 and CD5 positivity. There was complete absence of CD20 positive B lymphoid cell, suggesting the neoplastic nature of the lesion. Absence of CD30 excluded the possibility of CD30 positive lymphoproliferative disorders. In conjunction with morphology of small/medium-sized cells and CD4 positivity, a diagnosis of CD4 positive small/medium T cell lymphoma was given and confirmation by molecular testing was advised. On testing, TCRβ gene rearrangement by polymerase chain reaction confirmed the monoclonal nature of the lesion. Thorough physical examination and detailed laboratory and pathological evaluation was performed to exclude any underlying hematological malignancy. Chest X-ray, computed tomography of the neck and abdomen and whole body F-18 fludeoxyglucose positron emission tomography scan revealed no abnormalities. A final clinicopathological diagnosis of primary cutaneous CD4 positive small/medium T cell lymphoma was made. No further intervention was done.
 |
| Figure 1: Dense dermal periadnexal polymorphous mononuclear inflamatory infiltrate (H and E stain, ×100) |
 |
| Figure 2: Atypical polymporphous mononuclear inflamatory infiltrate (H and E stain, ×400) |
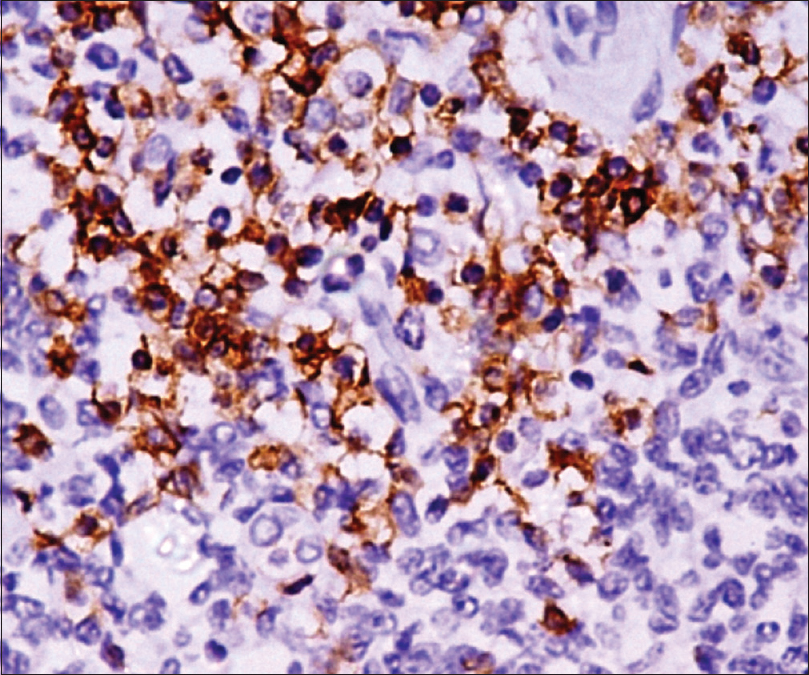 |
| Figure 3: Atypical cells showing positivity for imunohistochemical markers CD3 |
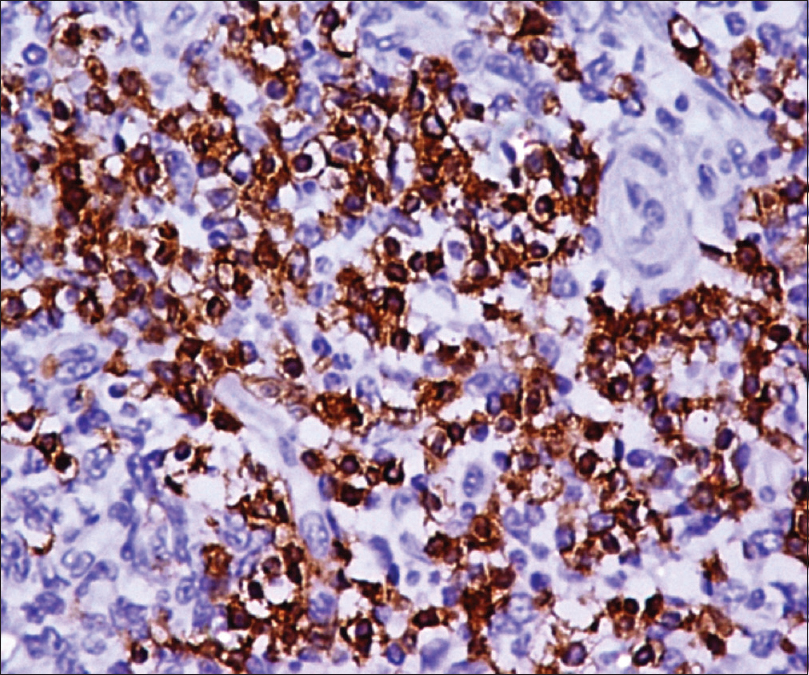 |
| Figure 4: Atypical cells showing positivity for imunohistochemical markers CD4 |
Primary cutaneous CD4 positive small/medium T cell lymphoma is a provisional entity in the 2005 world health organisation european organization for research and treatment of cancer classification for cutaneous lymphoma.[1] Usual presentation is in the form of localized disease with a solitary lesion in the head and neck region as observed in few reports describing the histopathological features of this disease in detail and predate the definition provided by the world health organisation european organization for research and treatment of cancer. Our findings are consistent with these published case reports.[2],[3],[4]
The differential diagnosis is difficult due to polymorphous nature and only mild to moderate cytological atypia in the lymphocyte population, in some cases to a degree that could occasionally be seen in reactive cutaneous T-cell infiltrates (pseudolymphoma). Moreover, phenotypic aberrancy may not be found always, hence confirmation of a clonal TCR gene rearrangement by molecular genetic analysis is often required for diagnosis. The histological features also show overlap with tumor phase mycosis fungoides and peripheral T-cell lymphoma, unspecified. In contrast to the band-like infiltrate with epidermotropism characteristic of mycosis fungoides, primary cutaneous CD4 positive small/medium T cell lymphoma shows a nodular infiltrate in the deep dermis and subcutis and does not significantly involve the epidermis. Cases with greater than 30% large pleomorphic tumor cells should be classified as peripheral T-cell lymphoma, unspecified, according to the definition provided by the world health organisation-european organization for research and treatment of cancer classification.[1] primary cutaneous CD4 positive small/medium T cell lymphoma has a favorable prognosis, with 5-year survival rates of 60–80%.[1] Meticulous careful review of literature explains variable survival rates due to heterogeneous spectrum of cases included in this entity.[5],[6] Elderly patients with large multiple lesions have unfavorable outcome despite aggressive radiotherapy and chemotherapy.[5] Our patient was elderly but presented with a solitary small lesion. Our follow-up of 10 months in the presented case was uneventful despite no further treatment offered after the final diagnosis. As observed by many authors, in a majority of this subset of patients, the lesion resolves with conservative therapy, and none of the reported patients died of the disease. In fact, few cases have shown spontaneous, partial, or complete resolution of the cutaneous lesion.[4],[7] We suggest that important poor predictors of outcome in this entity are large lesion size and multifocal presentation. Age does not appear to affect outcome in such cases, as evident by our case.
The recent demonstration that the neoplastic CD4+ T-cells in primary cutaneous-small medium T cell lymphoma coexpress PD1, BCL6, CXCL13, and ICOS has provided valuable insights into the potential derivation of primary cutaneous-small medium T cell lymphoma and its microenvironment from the follicular helper T-cells (TFH) found in reactive germinal centers.[6] In the times to come we may understand the pathogenesis of this entity in detail, which is needed for its apt classification and management.
In the largest case series of primary cutaneous small medium T cell lymphoma by Beltraminelli et al.,[8] the authors suggested that this entity be named “cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance” and should not be classified as benign or malignant.
In summary, primary cutaneous CD4 positive small/medium T cell lymphoma represents a rare but recognizable clinicopathological entity, and should be acknowledged as a diagnostic category separate from cutaneous peripheral T-cell lymphoma, unspecified. Given the histological and immunophenotypic overlap with benign and malignant cutaneous lymphoid infiltrates, diagnosis requires careful morphologic and immunophenotypic analysis, as well as molecular genetic studies. In patients with solitary lesions limited to the skin, an indolent clinical course after complete excision of the lesion can be expected. Recognition of this entity is important to avoid unnecessary treatment for these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85.
[Google Scholar]
|
| 2. |
Friedmann D, Wechsler J, Delfau MH, Estève E, Farcet JP, de Muret A, et al. Primary cutaneous pleomorphic small T-cell lymphoma. A review of 11 cases. The French Study Group on Cutaneous Lymphomas. Arch Dermatol 1995;131:1009-15.
[Google Scholar]
|
| 3. |
Kim YC, Vandersteen DP. Primary cutaneous pleomorphic small/medium-sized T-cell lymphoma in a young man. Br J Dermatol 2001;144:903-5.
[Google Scholar]
|
| 4. |
von den Driesch P, Coors EA. Localized cutaneous small to medium-sized pleomorphic T-cell lymphoma: A report of 3 cases stable for years. J Am Acad Dermatol 2002;46:531-5.
[Google Scholar]
|
| 5. |
Garcia-Herrera A, Colomo L, Camós M, Carreras J, Balague O, Martinez A, et al. Primary cutaneous small/medium CD4+ T-cell lymphomas: A heterogeneous group of tumors with different clinicopathologic features and outcome. J Clin Oncol 2008;26:3364-71.
[Google Scholar]
|
| 6. |
Lan TT, Brown NA, Hristov AC. Controversies and considerations in the diagnosis of primary cutaneous CD4+ small/medium T-cell lymphoma. Arch Pathol Lab Med 2014;138:1307-18.
[Google Scholar]
|
| 7. |
Bekkenk MW, Vermeer MH, Jansen PM, van Marion AM, Canninga-van Dijk MR, Kluin PM, et al. Peripheral T-cell lymphomas unspecified presenting in the skin: Analysis of prognostic factors in a group of 82 patients. Blood 2003;102:2213-9.
[Google Scholar]
|
| 8. |
Beltraminelli H, Leinweber B, Kerl H, Cerroni L. Primary cutaneous CD4+ small-/medium-sized pleomorphic T-cell lymphoma: A cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance? A study of 136 cases. Am J Dermatopathol 2009;31:317-22.
[Google Scholar]
|
Fulltext Views
3,317
PDF downloads
1,909





