Translate this page into:
Primary cutaneous cribriform apocrine carcinoma: Case report and literature review
2 Department of Surgery, Chia-Yi Christian Hospital, Health Sciences and Management, Chiayi, Taiwan
3 Department of Pathology; Department of Nursing, Chung-Jen College of Nursing, Health Sciences and Management, Chiayi, Taiwan
Correspondence Address:
Kai-Sheng Liao
Department of Pathology, Chia-Yi Christian Hospital, Chiayi
Taiwan
| How to cite this article: Wu JD, Changchien CH, Liao KS. Primary cutaneous cribriform apocrine carcinoma: Case report and literature review. Indian J Dermatol Venereol Leprol 2018;84:569-572 |
Abstract
Primary cutaneous cribriform apocrine carcinoma is a rare but distinct variant of primary cutaneous apocrine carcinoma and it is considered a low grade malignancy. We herein present a case of primary cutaneous cribriform apocrine carcinoma at the neck of a 26-year-old female. The tumor features a relatively well-circumscribed border and multiple aggregations of mildly pleomorphic epithelial cells with large ovoid nuclei, small nucleoli and abundant eosinophilic cytoplasms. Cribriform and tubular structures are the major architectural patterns. The primary differential diagnosis is cutaneous metastasis from a cribriform visceral carcinoma; others include primary secretory carcinoma of the skin, adenoid cystic basal cell carcinoma and primary cutaneous adenoid cystic carcinoma.
Introduction
Primary cutaneous cribriform apocrine carcinoma is considered as a rare but distinct variant of primary cutaneous apocrine carcinoma. Cribriform and tubular structures are the predominant patterns of such an entity, unlike the usual patterns described in primary cutaneous apocrine carcinoma. The primary differential diagnoses may include metastatic carcinoma (especially of the breast), primary cutaneous adenoid cystic carcinoma, and primary cutaneous secretory carcinoma. Prognostically, primary cutaneous cribriform apocrine carcinoma is considered as a low grade malignancy in which recurrence or metastasis after complete resection has never been reported in the literature.
Case Report
A 26-year-old female with no significant medical history presented with a 1 × 1-cm skin nodule on the right side of the neck, which had been noted for months. Irritation developed after self-application of some unknown ointment. The skin nodule (pointed by arrow) had an eczematous appearance and a firm consistency on physical examination [Figure - 1]a. Excision was performed and the specimen was sent for pathologic diagnosis. The small raised papules surrounding the nodule were considered acne clinically, and no treatment was given then.
 |
| Figure 1: Histopathologic features of the primary cutaneous cribriform apocrine carcinoma. (a):At scanning magnification, the neoplasm appears as a well-circumscribed, nonencapsulated dermal neoplasm. Lymphoid aggregates are present at the periphery of the neoplasm (H&E, ×40) (b): At higher magnification, epithelial aggregations are seen to be punctuated by small round spaces resulting in a cribriform or microcystic pattern (H&E, ×200) |
On microscopic examination, the nodule was found to be a relatively well-demarcated nonencapsulated tumor in the dermis [Figure - 1]b. It was composed of multiple nests of mildly pleomorphic epithelial cells with enlarged ovoid nuclei, small nucleoli, and abundant eosinophilic cytoplasm. Cribriform and tubular structures were the major architectural patterns. Scanty eosinophilic substance could be identified in some of the lumina in the areas displaying the cribriform and tubular architecture. Apocrine differentiation, i.e., decapitation secretion, was noted in some of the tubules at the periphery of the tumor. The stroma was mildly myxoid. There was no tumor necrosis. Lymphovascular and perineural invasions were not identified either.
The primary histopathological differential diagnoses included primary cutaneous cribriform apocrine carcinoma, primary cutaneous adenoid cystic carcinoma, primary cutaneous secretory carcinoma, and metastatic carcinoma.
Immunohistochemically, the tumor cells were reactive for cytokeratin 7, epithelial membrane antigen, S100, and CD117 [Figure - 2]a-d], while non-reactive for cytokeratin 20, carcinoembryonic antigen, smooth muscle actin, caudal-related homeobox gene 2, estrogen receptor, progesterone receptor, GATA transcription factor 3 (GATA is DNA sequence), and thyroid transcription factor 1. The results of mucicarmine and periodic acid-Schiff (PAS) staining were also negative. Alcian blue (pH 2.5) stain was positive in the stroma, but negative in the eosinophilic substance as well as in the cytoplasm of the tumor cells [Figure - 2]e.
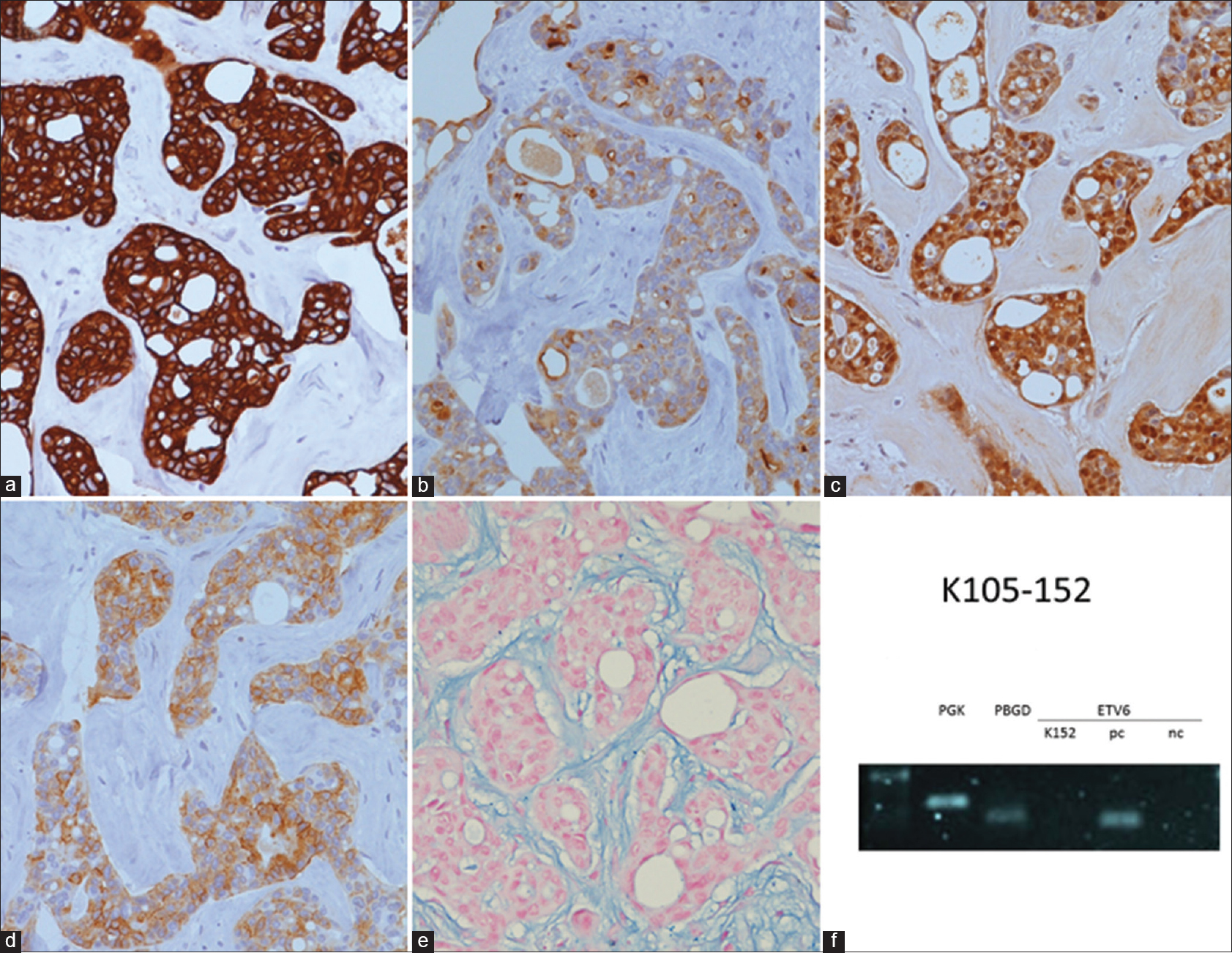 |
| Figure 2: Immunostains, histochemical stain, and molecular testing of the primary cutaneous cribriform apocrine carcinoma. (a): The tumor cells are immunoreactive for cytokeratin 7 (×400). (b):The tumor cells are immunoreactive for epithelial membrane antigen (×400) (c)The tumor cells are immunoreactive for S100 (×400) (d): The tumor cells are immunoreactive for CD117 (cytoplasmic and membranous staining) (×400) (e): The stroma is positively stained by alcian blue (pH 2.5) stain (×400) (f): The result is negative for the detection of ETV6-NTRK3 fusion transcript via RT-PCR technique. The bands of PGK gene and PBGD gene were shown to test the quality of the extracted RNA. pc: Positive control, nc: Negative control, K152: Present case |
After excluding the evidence of breast and visceral malignancy, the diagnosis of primary cutaneous cribriform apocrine carcinoma was made, based mainly on the histopathologic and immunohistochemical features.
Discussion
Primary cutaneous cribriform apocrine carcinoma is considered as a distinct variant of primary cutaneous apocrine carcinoma. Based on the literature review, all the tumors with such a diagnosis are mostly dermal and nonencapsulated.[1] The tumors are relatively symmetric and well-circumscribed, while some may have jagged borders. There is no connection to the epidermis. Solid aggregations of epithelial cells are observed, within which there are small round spaces with tubular and cribriform patterns. Cuboidal or cylindrical lining cells with focal decapitation secretion can be identified in the lumina of the tubules. Eosinophilic homogeneous substance can also be found in some of the lumina.
The tumor cells typically bear enlarged, round to oval, hyperchromatic and pleomorphic nuclei with inconspicuous or absent nucleoli. They usually have scant and eosinophilic cytoplasm, and mitotic figures are sparse. Individual necrotic cells or foci of necrosis are occasionally present. The stroma is typically scanty and composed of collagen bundles. There may be nodular lymphoid aggregates at the periphery, some with lymphoid follicle formation. There is no perineural or lymphovascular invasion throughout the reported lesions.
Immunohistochemically, the tumor cells express cytokeratin AE1/AE3, Cam 5.2 (clone name of the anti-cytokeratin antibody, which is reactive to cytokeratin 8), cytokeratin 7, epithelial membrane antigen, and carcinoembryonic antigen. The results of cytokeratin 20, gross cystic disease fluid protein 15, S100, smooth muscle actin, muscle specific antigen, and calponin stains are negative.[1] The immunoprofile of this present case differs slightly in that the result of S100 stain is positive and the result of carcinoembryonic antigen stain is negative. However, this minor discrepancy does not preclude the diagnosis of primary cutaneous cribriform apocrine carcinoma because we believe the histopathologic features play a more significant role.
In the literature, the primary differential diagnoses of primary cutaneous cribriform apocrine carcinoma include cutaneous metastasis from a cribriform visceral carcinoma and primary cutaneous adenoid cystic carcinoma.[1]
Obtaining the previous medical history of breast or visceral carcinoma is crucial to exclude the possibility of cutaneous metastasis.
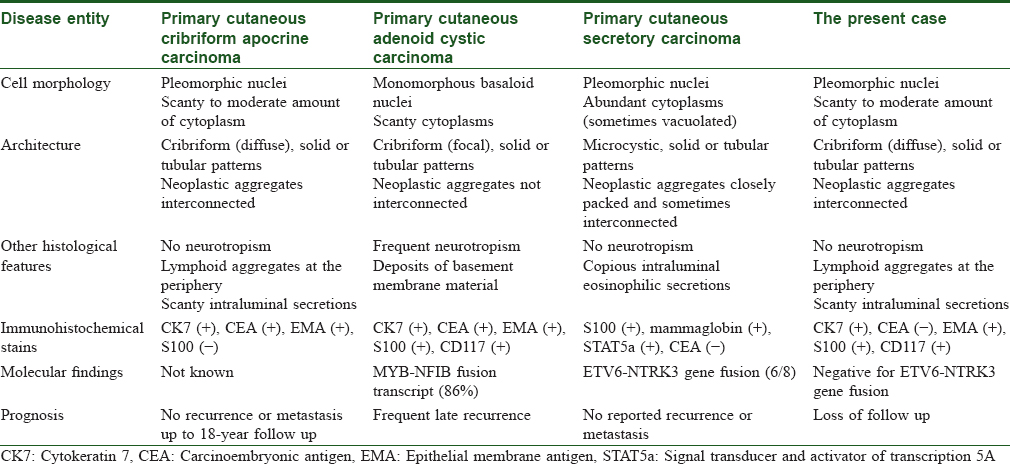
Primary cutaneous cribriform apocrine carcinoma differs from adenoid cystic carcinoma in cell morphology, presence of neurotropism, and deposition of basement membrane material [Table - 1].[1],[2],[3],[4],[5],[6],[7] The tumor cells of the latter are more monotonous and basophilic. Neurotropism is also frequently seen in the latter, as well as deposition of basement membrane material.
CD117 staining was performed in our case with the initial intent of ruling out the diagnosis of adenoid cystic carcinoma. CD117 positivity in the present case prompted us to search the literature, which revealed that some cases of apocrine carcinoma, as well as other benign and malignant sweat gland tumors, can also display positive CD117 stain.[6],[7] So, CD117 stain might not be helpful in differentiating apocrine carcinoma and adenoid cystic carcinoma.
Another important differential diagnosis is primary secretory carcinoma of the skin. The characteristic balanced t(12;15) (p13;q25) ETV6-NTRK3 translocation can be observed in some cases of the primary cutaneous secretory carcinoma.[2],[3],[6],[8] Regarding our case, the ETV6-NTRK3 fusion transcript was not detected via the reverse transcription polymerase chain reaction technique [Figure - 2]f. However, failure to detect such genetic fusion does not preclude the diagnosis of primary cutaneous secretory carcinoma. According to the reports in the literature, we have concluded that this entity differs from primary cutaneous cribriform apocrine carcinoma in at least three aspects on H and E stain [Table - 1].[2],[3],[6],[8] First, primary cutaneous secretory carcinoma has a less well-circumscribed border. Secondly, it has more, if not copious, eosinophilic or amphophilic intraluminal secretions. Thirdly, some of the tumor cells possess vacuolated cytoplasm, which is not observed in the latter. Immunostains appears of limited help in differentiating these two entities.
Longstanding pre-existent benign apocrine lesions (including hyperplasia, cystadenoma, cylindroma, syringocystadenoma papilliferum, and tubular adenoma) may be evident in a primary cutaneous apocrine carcinoma, raising the possibility of a malignant transformation.[5] Such a phenomenon was not observed in our case.
As none of the reported cases recurred or metastasized in the follow-up (up to 18 years), it may be debatable whether this entity is a benign or malignant neoplasm. Based on several reasons, including the jagged border, nuclear pleomorphism, occasional necrosis, and DNA aneuploidy, the authors still regard this entity as a low grade malignancy.[1]
We herein present a case of primary cutaneous cribriform apocrine carcinoma on the neck. The morphological features are key to such a rare diagnosis. Complete excision is adequate treatment.
Acknowledgment
We would like to thank Dr. Huang HY, Chief of Department of Pathology, Kaohsiung Chang Gung Memorial Hospital, for his generous assistance of performing reverse transcription polymerase chain reaction of ETV6-NTRK3fusion transcript on this case.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Rütten A, Kutzner H, Mentzel T, Hantschke M, Eckert F, Angulo J, et al. Primary cutaneous cribriform apocrine carcinoma: A clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol 2009;61:644-51.
[Google Scholar]
|
| 2. |
Brandt SM, Swistel AJ, Rosen PP. Secretory carcinoma in the axilla: Probable origin from axillary skin appendage glands in a young girl. Am J Surg Pathol 2009;33:950-3.
[Google Scholar]
|
| 3. |
Kazakov DV, Hantschke M, Vanecek T, Kacerovska D, Michal M. Mammary-type secretory carcinoma of the skin. Am J Surg Pathol 2010;34:1226-7.
[Google Scholar]
|
| 4. |
McKee PH, Calonje JE, Brenn T, Lazar A. Tumors of the sweat glands. McKee's Pathology of the Skin. 4th ed., Ch. 33. Philadelphia, USA: Elsevier Saunders; 2011. p. 1516-8.
[Google Scholar]
|
| 5. |
Ramakrishnan R, Chaudhry IH, Ramdial P, Lazar AJ, McMenamin ME, Kazakov D, et al. Primary cutaneous adenoid cystic carcinoma: A clinicopathologic and immunohistochemical study of 27 cases. Am J Surg Pathol 2013;37:1603-11.
[Google Scholar]
|
| 6. |
Nishida H, Daa T, Kashima K, Arakane M, Urabe S, Yoshikawa Y, et al. KIT (CD117) expression in benign and malignant sweat gland tumors. Am J Dermatopathol 2015;37:898-905.
[Google Scholar]
|
| 7. |
Lee SJ, Yang WI, Kim SK. Primary cutaneous adenoid cystic carcinoma arising in umbilicus. J Pathol Transl Med 2016;50:322-4.
[Google Scholar]
|
| 8. |
Bishop JA, Taube JM, Su A, Binder SW, Kazakov DV, Michal M, et al. Secretory carcinoma of the skin harboring ETV6 gene fusions: A cutaneous analogue to secretory carcinomas of the breast and salivary glands. Am J Surg Pathol 2017;41:62-6.
[Google Scholar]
|
Fulltext Views
5,233
PDF downloads
2,160





