Translate this page into:
Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia
2 Dermatopathology Section, Boston University School of Medicine, Boston, Massachusetts 02118,
Correspondence Address:
M Mahalingam
609 Albany Street, J-301, Dermatopathology section, Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118
| How to cite this article: Konda S, Beckford A, Demierre M F, Mahalingam M. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol 2011;77:314-317 |
Abstract
Primary cutaneous malignancies arising in association with chronic lymphocytic leukemia (CLL) are notable for their atypical clinical and histological presentation. We report a 69-year-old man with a 17-year history of CLL who presented for evaluation of a well-defined red to violaceous nodule with a central depressed scar on the left lower extremity. Microscopic examination of a punch biopsy revealed an infiltrate of predominantly small lymphocytes with scattered large, atypical epithelioid cells. Immunohistochemical stains revealed diffuse positive staining of the lesional cells with CD20+ and bcl-6+ and focal positive staining with bcl-2+ (negative CD10 and CD23), findings which, in conjunction with the histology, were most compatible with a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). A review of the clinical charts revealed several prior biopsies with varied diagnoses. In light of the most recent biopsy findings, all previous biopsies were re-reviewed and interpreted as PCFCL arising in the setting of CLL. Features contributing to the diagnostic conundrum in this case included an atypical clinical and histological presentation, lack of pertinent clinical history and multiple presentations at different institutions.Introduction
Primary cutaneous follicle center lymphoma (PCFCL) manifests predominantly in middle-aged adults and characteristically presents with solitary or grouped erythematous to violaceous plaques, nodules or tumors, usually localized to the head or trunk. We report a case of PCFCL in a patient with a longstanding history of chronic lymphocytic leukemia (CLL). The patient had previously presented to multiple institutions with varied diagnoses. To the best of our knowledge, this is the first documented case of a PCFCL arising in the setting of CLL.
Case Report
A 69-year-old man with a 17-year history of CLL presented in September 2008 with a gradually increasing, non-tender lesion on his left lower extremity. His clinical history was significant as he had primary B-cell lymphoma diagnosed ten years prior to the current presentation on the same extremity. The lesions had been managed with intralesional kenalog (ILK) and topical steroids with documented regression. During the course of the last several months, the patient noticed progressive multifocal non-ulcerating lesions on his left lower extremity, which were again treated with ILK. His past medical history was significant for hypertension, hyperlipidemia, hypergammaglobulinemia and CLL. The CLL was Rai Stage I and had not required any treatment with white blood cell (WBC) counts ranging from 50-60 Χ 10 3 /mm 3 without anemia, thrombocytopenia, lymphadenopathy or hepatosplenomegaly. There was no history of a previous exposure to ionizing radiation or cytotoxic agents and he had a 71-year-old sister who was diagnosed with CLL six years ago.
On examination, the medial third of his left lower extremity had a 2.2 Χ 2 Χ 1 cm well-defined red to violaceous nodule with a central healed scar [[Figure - 1]a]. His popliteal fossa had multiple well-defined mildly erythematous annular lesions up to 5 cm in diameter with raised edges [[Figure - 1]b]. Microscopic examination of a punch biopsy of the nodule revealed a superficial and deep, perivascular and interstitial infiltrate of predominantly small lymphocytes with scattered large atypical epithelioid cells [[Figure - 1]c,d]. Immunohistochemical stains revealed diffuse positive staining of the lesional cells with CD20 and bcl-6 [[Figure - 1]e,f], focal positive staining with bcl-2 and, negative staining with CD10 and CD23. This immunoprofile, in conjunction with the histological findings, was consistent with a B-cell lymphoma of follicle center cell origin. The complexity of the clinical history warranted a clear review of all previous slides [Table - 1] [Figure - 2] and [Figure - 3].

 |
| Figure 1: September 2008 Well-defined red to violaceous nodule with a central healed scar (a) and popliteal fossa with multiple well-defined mildly erythematous annular lesions with raised edges (b). Histopathology of the nodular lesion showing an infiltrate of predominantly small lymphocytes with scattered large atypical epithelioid cells (c= H and E, ×4 and d= H and E, ×20) and immunohistochemical stains showing diffuse positive staining of lesional cells (e= CD20, ×4 and f= bcl-6, ×4). Diagnosis at our institution was follicle center cell lymphoma |
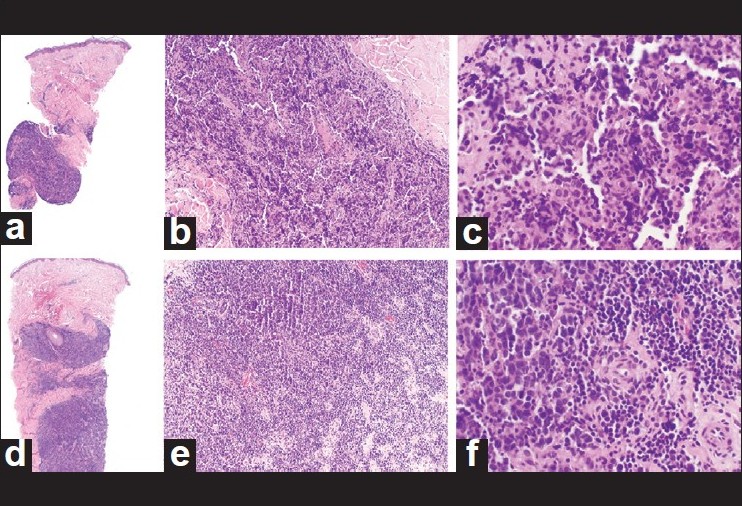 |
| Figure 2: June and November 2003 Prior histopathology from June 2003 showing perivascular aggregates of small lymphoid cells with occasional large cells. Initial diagnosis at outside institution was CLL. a= June 2003 (H and E, ×4); b= June 2003 (H and E, ×10); c= June 2003 (H and E, ×20). Prior histopathology from November 2003 showing large atypical cells with oval, irregular or indented nuclei and scant cytoplasm in the deep dermis. Initial diagnosis at outside institution was diffuse large B-cell lymphoma. d= November 2003 (H and E, ×4); e= November 2003 (H and E, ×10); f= November 2003 (H and E, ×20) |
 |
| Figure 3: August 2006 and August 2008 Prior histopathology from August 2006 showing small lymphoid cells with angulated and cleaved nuclei. Initial diagnosis at outside institution was follicular lymphoma. a= August 2006 (H and E, ×4); b= August 2006 (H and E, ×10); c= August 2006 (H and E, ×20). Prior histopathology from August 2008 showing small lymphoid cells with angulated and cleaved nuclei. Initial diagnosis at outside institution was follicle center cell lymphoma. d= August 2008 (H and E, ×4); e= August 2008 (H and E, ×10); f= August 2008 (H and E, ×20) |
His current WBC count was 60.3 Χ 10 3 /mm 3 and a lymphoma panel from his peripheral blood was notable for a monoclonal B-cell population expressing CD19, CD5, CD23 and kappa light chain consistent with CLL. Flow cytometric analysis of a bone marrow aspirate specimen revealed a monoclonal population of B-cells expressing CD19, CD5, CD23, and kappa light chain immunoglobulins, consistent with involvement of bone marrow by CLL. Whole-body Positron Emission Tomography--Computed Tomography scan revealed a 1.9 cm subcutaneous nodule in the anteromedial soft tissues of the left thigh with marked increased radiotracer uptake. There was also low-level activity within the subcutaneous region just below the left calf in the medial soft tissues. He was started on chemotherapy, after four cycles of which his PCFCL and CLL appeared to be in remission with a normalized WBC count.
Discussion
CLL occurs at the stage of activated memory B-cells, and follicle center cell lymphoma occurs at the subsequent stage of mature memory B-cells. [1] This can pose a diagnostic dilemma as they share similar immunohistochemical markers [Table - 2]. Uckun studied the immunophenotypic features of normal B-cell precursors and reported that expression of CD10 and CD19 usually precedes the acquisition of CD20, CD21, CD22, CD23, CD24, sIgD, and sIgM. [2]

Furthermore, the neoplastic cells in PCFCL consistently express bcl-6 which may explain why the immunophenotype in the November 2003 biopsy in our case was concerning for diffuse large B-cell lymphoma. [3] In PCFCL, the t(14;18)(q32;q21) is typically absent. [4] Thus, genetic investigations for this translocation were not performed on our patient. Nonetheless, the biopsy at our institution in September 2008 demonstrated small clusters of bcl-6+ cells outside neoplastic bcl-2+ follicle cells, a finding virtually diagnostic of follicle center cell lymphoma. While it is unusual for a secondary B-cell malignancy, PCFCL in this case, to develop when the immunocompromised status of patients with CLL is largely secondary to B-lymphocyte defects, it has been hypothesized that profound T-cell dysregulation may contribute to the survival and perpetuation of malignant B-cells in these patients. [5]
Prognosis of PCFCL is excellent, with a five-year survival rate greater than 95% even though local cutaneous relapses may develop in 20% of patients and extracutaneous dissemination may occur in 5-10% of patients. [6] However, when PCFCL is localized to the lower legs, as in our patient, recurrences may be more frequent with a considerably worse prognosis. [7] Cytological grade or growth pattern does not appear to have an impact on prognosis. Radiation is highly effective with few side-effects and a median disease-free period ranging from 15.5 to 22.03 months. [6],[8] Surgical excision for small, isolated lesions achieves clinical remission in most patients and less than half develop a cutaneous relapse. [7] Intralesional interferon-alpha has been reported with documented clinical remission; however, efficacy is indeterminate because of rather short follow-up periods. [7] Intralesional or systemic rituximab, a monoclonal antibody directed against the CD20 antigen, may also be helpful as monotherapy in the management of PCFCL on the leg. [9] Rituximab in combination with chemotherapy improves overall survival compared to chemotherapy alone in patients receiving induction and maintenance therapy for newly diagnosed or relapsed PCFCL. [10] This treatment regimen can also be an effective induction therapy for CLL, as documented in our patient.
Our case highlights problems associated with leukemic infiltrates from CLL confounding the diagnosis of primary cutaneous lymphoid dyscrasias. The spectrum of B-lineage antigen acquisition can often be problematic in determining the precise immunophenotype of B-cell malignancies when malignant clones arise in adjacent stages of B-cell development, as illustrated in our case. Confounding issues were multi-institutional patient care. Our patient had skin biopsies performed and analyzed at four different institutions before presenting to our clinic. The lack of communication between individual medical providers and the difficulty in obtaining prior skin biopsy results were contributing factors in the delay of this patient′s correct and efficient diagnosis.
| 1. |
Blom B, Spits H. Development of human lymphoid cells. Ann Rev Immunol 2006;24:287-320.
[Google Scholar]
|
| 2. |
Uckun FM. Regulation of human B-cell ontogeny. Blood 1990;76:1908-23.
[Google Scholar]
|
| 3. |
Hoefnagel JJ, Dijkman R, Basso K, Jansen PM, Hallermann C, Willemze R, et al. Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood 2005;105:3671-8.
[Google Scholar]
|
| 4. |
Streubel B, Scheucher B, Valencak J, Huber D, Petzelbauer P, Trautinger F, et al. Molecular cytogenetic evidence of t(14;18)(IGH;BCL2) in a substantial proportion of primary cutaneous follicle center lymphomas. Am J Surg Pathol 2006;30:529-36.
[Google Scholar]
|
| 5. |
Scrivener S, Kaminski ER, Demaine A, Prentice AG. Analysis of the expression of critical activation/interaction markers on peripheral blood T cells in B-cell chronic lymphocytic leukaemia: Evidence of immune dysregulation. Br J Haematol 2001;112:959-64.
[Google Scholar]
|
| 6. |
Piccinno R, Caccialanza M, Berti E. Dermatologic radiotherapy of primary cutaneous follicle center cell lymphoma. Eur J Dermatol 2003;13:49-52.
[Google Scholar]
|
| 7. |
Senff NJ, Noordijk EM, Kim YH, Bagot M, Berti E, Cerroni L, et al. European organization for research and treatment of cancer and international society for cutaneous lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 2008;112:1600-9.
[Google Scholar]
|
| 8. |
Pimpinelli N, Santucci M, Bosi A, Moretti S, Vallecchi C, Messori A, et al. Primary cutaneous follicular centre-cell lymphoma--a lymphoproliferative disease with favourable prognosis. Clin Exp Dermatol 1989;14:12-9.
[Google Scholar]
|
| 9. |
Bonnekoh B, Schulz M, Franke I, Gollnick H. Complete remission of a primary cutaneous B-cell lymphoma of the lower leg by first-line monotherapy with the CD20-antibody rituximab. J Cancer Res Clin Oncol 2002;128:161-6.
[Google Scholar]
|
| 10. |
Vidal L, Gafter-Gvili A, Leibovici L, Shpilberg O. Rituximab as maintenance therapy for patients with follicular lymphoma. Cochrane Database Syst Rev 2009;2:CD006552.
[Google Scholar]
|
Fulltext Views
2,645
PDF downloads
2,047





