Translate this page into:
Primary dapsone resistant Mycobacterium leprae in a non endemic country
2 Department of Dermatology, Hospital Kuala Lumpur, Jalan Pahang, Kuala Lumpur, Malaysia
3 Leprosy Unit, National Public Health Laboratory Sungai Buloh, Selangor, Malaysia
4 Department of Family Medicine, University Kebangsaan Malaysia Medical Center, Bandar Tun Razak, Cheras, Malaysia
Correspondence Address:
Adawiyah Jamil
Department of Medicine, University Kebangsaan Malaysia Medical Center, Bandar Tun Razak, Cheras- 56000 Kuala Lumpur
Malaysia
| How to cite this article: Jamil A, Noor NM, Osman AS, Baseri MM, Muthupalaniappen L. Primary dapsone resistant Mycobacterium leprae in a non endemic country. Indian J Dermatol Venereol Leprol 2013;79:527-529 |
Sir,
Dapsone monotherapy in leprosy resulted in high resistance rates since the 1960s. After introduction of multidrug therapy (MDT), the rate declined but dapsone resistant Mycobacterium leprae has not been eradicated. [1],[2],[3],[4],[5] Leprosy is not endemic in Malaysia, however, surveillance of drug resistance was continued to guide therapy to prevent the spread of resistant strains. We report a 12 years surveillance of primary dapsone resistance using the mouse foot pad (MFP) technique.
Skin biopsy samples of multibacillary leprosy patients throughout the country were sent to the National Public Health Laboratory, Sungai Buloh. The MFP cultivation technique: Saline albumin is added to grounded
4 mm × 12 mm skin specimen containing lesion to produce a 10 4 bacilli/0.03 ml suspension. The suspension is inoculated into mice foot pads. The mice were given feeds mixed with dapsone concentrations of 0.01% or 0.001% or 0.0001%. Six mice were fed with each concentration. A control group of six mice was not treated. Reassessment of the inoculated M. leprae was performed by harvesting the footpad of one mouse from each group after six months, the rest of the mice were assessed after 12 months incubation. Results are expressed as low level (resistance to dapsone concentration 0.0001%), intermediate (resistance to concentration 0.001%) or high level resistance (resistance to concentration 0.01%). Results from 1997 to 2008 were retrospectively reviewed.
A total of 457 cases were referred to the laboratory, 362 were newly diagnosed multibacillary leprosy patients. Ninety five were patients with relapsed disease or treatment problems. The median age was 37(6-85) years, 290(80.1%) were males and 72(19.9%) were females. There were 217(59.9%) Malaysians, 125(34.5%) Indonesians, 5(1.4%) Myanmarese, 5(1.4%) Nepalese, and 2(0.6%) Bangladeshis. Specimens from 271(74.9%) patients were successfully cultured and tested. Overall, 60(22.1%) were identified to have intermediate and high level resistance. Low level resistance was observed in 83(31%) cases. The prevalence of resistance is illustrated in [Table - 1].
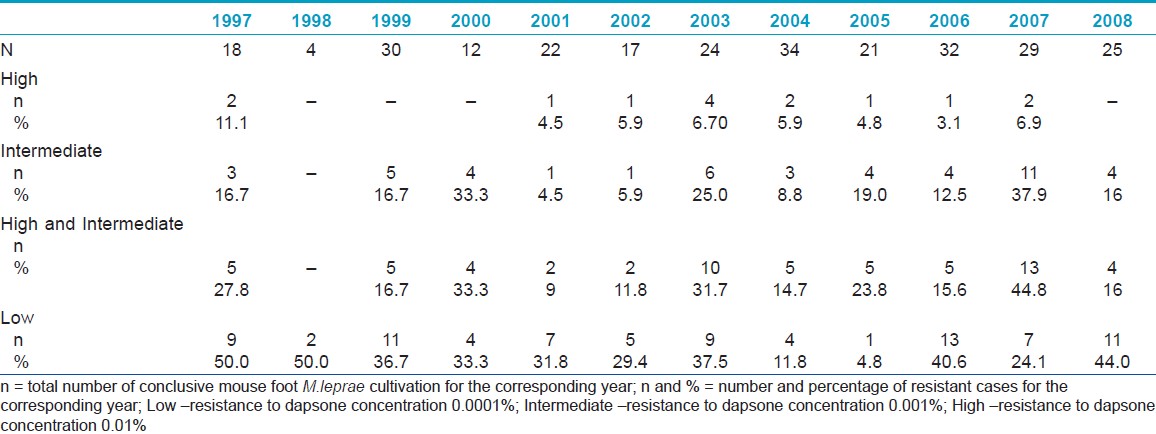
Intermediate and high-level resistances account to about 10-20%; however, in 2000, 2003 and 2007, the prevalence was higher than 30%. The highest clinically significant resistance recorded was 44.8% in 2007, of which 6.9% was of high level. In cases with intermediate or high resistance, the majority of patients are Malaysians (66%), followed by Indonesians (27%), Myanmarese (3.4%), and Nepalese (1.7%). Low level resistance was not observed in the Indonesians, 63.6% had intermediate or high level resistance.
Our results showed a high prevalence of low level resistance, however, this is not associated with clinical therapy failure as the dose used in the MFP is equivalent to treating a patient with dapsone 1mg daily, compared to the standard dose of 100 mg daily. [1],[2] Intermediate and high level resistances are clinically significant.
There are a few reported prevalence of primary dapsone resistant M. leprae from this region. Kai et al.,[3] in Vietnam found 6.1% folP1 mutation 33 new cases of leprosy and 6.4% in recently diagnosed cases between 2004 and 2009. In Yangoon, Myanmar between 2003 and 2005, there were 7.3% folP1 mutation in 54 new/recent cases. [2] In Cebu Philippines (2001-2006), 2.6% folP1 mutation was seen in 77 new/recent cases. [2] Between 2000-2005, 0.8% folP1 mutation was reported in Indonesia (North Maluku, North Sulawesi). [2] Cambau et al.,[1] in 1987-2000, found 14.3% folP1 mutation and equal number of resistance using the MFP in 21 new/recent cases in patients from the West Indies, Africa, Asia, and the Pacifics. In the 1980s, de la Cruz et al.,[4] detected 21% dapsone resistance using the MFP in 38 new cases in Cebu, Philippines.
Our figures within the same period are higher compared to other countries. Although, in general, the prevalence is much lower compared to pre-MDT, dapsone resistance persisted. Current dapsone resistant M. leprae in Malaysia is likely acquired from patients with resistant strains in the pre-MDT era. Transmission of dapsone resistant M. leprae among close contacts has been demonstrated by Li et al.,[5] in a molecular epidemiological study. Irregular therapy, inadequate coverage, and delay in diagnosis or treatment are other reasons why resistance continues.
M. leprae cultivation using MFP is currently the standard in drug susceptibility testing. However, the MFP is time consuming, labor intensive, and insensitive. Detection of mutation in the folP1 gene identifies dapsone resistance more rapidly, but compared to DNA methods, MFP differentiates viable and non-viable M. leprae and it reflects clinical efficacy. [1]
Primary dapsone-resistance in Malaysia may increase further. Early identification of resistance using rapid methods of detection will help maintain the effectiveness of the MDT and prevent spread of resistant cases. M. leprae cultivation using MFP may be used in adjunct to molecular techniques.
| 1. |
Cambau E, Carthagena L, Chauffour A, Ji B, Jarlier V. Dihydropteroate synthase mutations in the folP1 gene predictdapsone resistance in relapsed cases of leprosy. Clin Infect Dis 2006;42:238-41.
[Google Scholar]
|
| 2. |
Matsuoka M, Budiawan T, Aye KS, Kyaw K, Tan EV, Cruz E, et al. The frequency of drug resistance mutations in Mycobacterium lepraeisolates in untreated and relapsed leprosy patients from Myanmar, Indonesia and the Philippines. Lepr Rev 2007;78:343-52.
[Google Scholar]
|
| 3. |
Kai M, NguyenPhuc NH, Nguyen HA, Pham TH, Nguyen KH,Miyamoto Y, et al.Analysis of drug-resistant strains of Mycobacterium leprae in an endemic area of Vietnam. Clin Infect Dis 2011;52:e127-32.
[Google Scholar]
|
| 4. |
de la Cruz E, Cellona RV, Balagon MV, Villahermosa LG, Fajardo TT Jr., Abalos RM,et al. Primary dapsone resistance in Cebu, The Philippines; cause for concern. Int J Lepr Other Mycobact Dis 1996;64:253.
[Google Scholar]
|
| 5. |
Li W, Sakamuri RM, Lyons DE, Orcullos FM, Shinde V, de la Pena EL, et al. Transmission of dapsone-resistant leprosy detected by molecular epidemiological approaches. Antimicrob Agents Chemother 2011;55:5384-7.
[Google Scholar]
|
Fulltext Views
2,573
PDF downloads
1,501





