Translate this page into:
Primary drug resistant cutaneous tuberculosis: A retrospective case series of seven patients
Corresponding author: Dr. Parul Verma, Department of Dermatology, Venereology & Leprosy, King George’s Medical University, Lucknow, Uttar Predesh, India. parulverma_30@yahoo.co.in
-
Received: ,
Accepted: ,
Read CORRECTED-ARTICLE associated with this - 10.25259/IJDVL_63_2023-ER
How to cite this article: Shukla P, Verma P, Suvirya S, Jain A, Singh U, Singh S, et al. Primary drug resistant cutaneous tuberculosis: A retrospective case series of seven patients. Indian J Dermatol Venereol Leprol. 2025;91:S21-S23. doi: 10.25259/IJDVL_63_2023
Dear Editor,
India accounts for a large burden of the total tuberculosis (TB) cases in the world and at least half a million subsequent deaths annually.1 Cutaneous tuberculosis (CTB) affects <1–2% of the extra-pulmonary tuberculosis cases. Multi-drug resistant (MDR) TB cases are on the rise and are painting a troublesome situation globally. Multidrug resistant (MDR) cutaneous tuberculosis was first reported in 1995 and since then isolated case reports and small series have been reported. We present a case series of 7 patients with drug-resistant cutaneous tuberculosis.
Patients diagnosed with cutaneous tuberculosis between 2019 and 2022 at a tertiary care center in North India were included in this series. Clinical data and laboratory reports that included chest X-ray, Mantoux test, histopathology, culture, Gene-Xpert and drug susceptibility testing (DST) results, and other relevant reports where available were retrieved.
A total of seven out of 54 patients with CTB were diagnosed as Drug resistant (DR-TB) in the last 4 years. All the cases were HIV negative. Three patients had lupus vulgaris, two had scrofuloderma, one had tubercular abscess, and one had tubercular chancre [Figures 1–3]. In six patients, resistance to rifampicin was subsequently managed with an Multidrug resistant (MDR)-TB regimen (all oral longer MDR regimen – bedaquiline for six months; levofloxacin, linezolid, clofazimine, cycloserine for 18–20 months). In one patient where isoniazid (INH) mono-resistance was detected, anti-tubercular therapy (ATT) was given as per the standard INH resistance protocol. Response was seen starting at 4 weeks, one patient was lost to follow up and others completed treatment with complete healing. Antitubercular treatment (ATT) was well tolerated, except in one patient where flu-like symptoms were troublesome for the initial 2–3 weeks. No significant lab abnormalities were noted [Table 1].
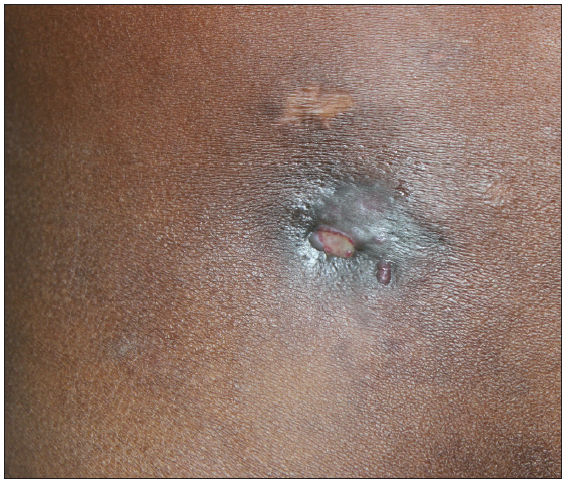
- Draining sinus above the right inguinal region in a 27-year-old male.
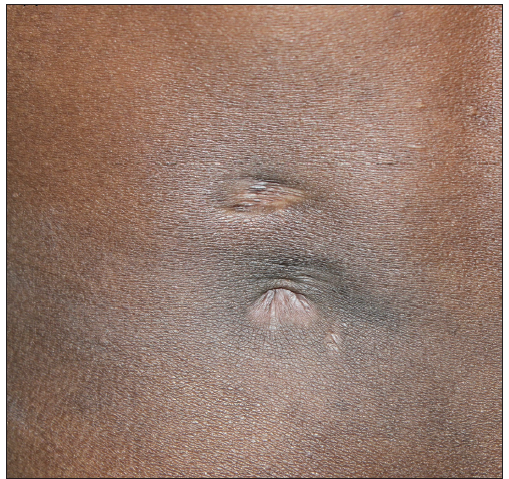
- Post-treatment healed sinus with puckering in the same patient.
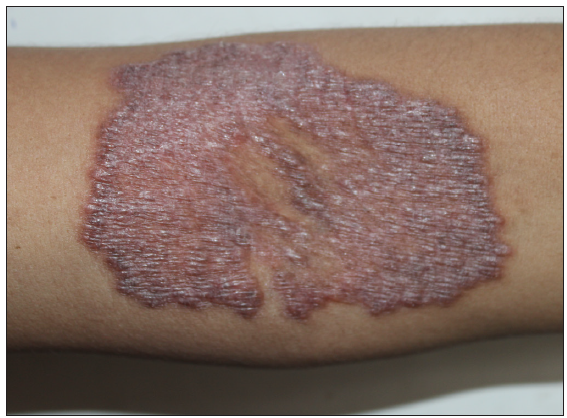
- A well-defined erythematous plaque over the forearm in a 26-year-old female.
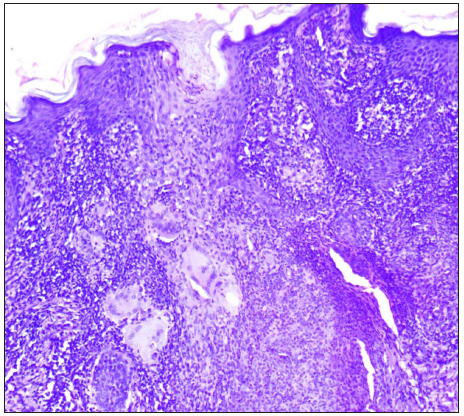
- Granulomas surrounded by epithelioid cells, lymphocytes, and giant cells (Haematoxylin and eosin, 100x).
| Age/sex | Diagnosis and duration | CB-NAAT | CB-NAAT (R) | Histopathology | Culture | Systemic involvement | Degree of M.tuberculosis detected in the test sample |
|---|---|---|---|---|---|---|---|
| 26/F | Lupus vulgaris/ 2 years | + | + | Well-formed granulomas surrounded by epithelioid cells | + Isoniazid/Rifampicin (HR) resistance | – | Low |
| 34/M | Lupus vulgaris/ 2 years | + | + | Granulomas surrounded by epitheloid cells | – | – | Very low |
| 27/M | Scrofuloderma/ 9 months | Tissue -, pus + | + | Caseous necrosis surrounded by granulomatous process | – | Very low | |
| 21/F | Scrofuloderma/ 1 year | + | + | Interface dermatitis | – | – | Very low |
| 42/F | Tubercular abscess/ 18 months |
Tissue _ Pus + |
+ | Central ulceration with caseous necrosis with surrounding giant cells | – | Disseminated TB | Very low |
| 27/M | Tubercular chancre/ 2 years | + | + | Mixed inflammatory vasculitis | Pulmonary TB + iliac bone involvement | Very low | |
| 28/F | Lupus vulgaris/ 6 years | _ | _ | Granulomas surrounded by epithelioid cells | Positive (DST isoniazid resistant) | – | Low |
CB-NAAT(R) denotes samples with resistance to rifampicin detected on CB-NAAT, DST: Drug susceptibility testing, CB-NAAT: Cartridge based nucleic acid amplification test, + means positive test for M.Tuberculosis, – means negative test for M.Tuberculosis.
MDR-TB is defined as TB caused by the strains of Mycobacterium tuberculosis that exhibit resistance to both rifampicin and isoniazid. As per WHO global reports, at least 4.1% of all newly detected cases of TB exhibit multi-drug resistance or rifampicin resistance.1 The WHO Global TB Programme aims at a world free of tuberculosis, where there are zero deaths and suffering due to the disease. However, increased incidence of DR-TB is the greatest obstacle towards this goal as it is associated with a higher risk of relapse, increased treatment failure, prolonged transmission of the bacilli, and even death.2
Reports suggest that almost 23% of the new cases in India exhibit resistance to any anti-tubercular drug, with the maximum resistance seen with isoniazid, followed by pyrazinamide and streptomycin.3 Higher bacillary load is considered a contributor to the development of DR-TB due to the increased risk of spontaneous mutations conferring resistance to one or more drugs.4 Risk factors for developing MDR-TB have been studied and those of significance include positive sputum acid-fast bacilli (AFB) smear, lung cavity, previously diagnosed TB, and previous history of taking ATT.5
The extra-pulmonary forms such as cutaneous tuberculosis are essentially pauci-bacillary and this makes the isolation of organisms in culture difficult. Drug susceptibility testing (DST) for TB can be performed using either the solid culture (Lowenstein–Jensen medium) or liquid culture (MGIT 960), molecular assays such as Gene-Xpert Mycobacterium tuberculosis complex and resistance to rifampin (MTB/RIF) assay and line probe assays (LPAs) which detect resistance quickly in a matter of hours. These newer tests have been introduced for rapid identification of rifampicin‐resistant MTB, which is considered a reliable proxy for MDR-TB. However, the commercial molecular tests detect only select resistance-conferring mutations and are currently unavailable for many second-line drugs. Also, whole-genome sequencing (WGS) of TB strains in India has demonstrated novel mutations which suggests that the TB strains circulating in our country are different from those in other parts of the world and so may not be detected by the commonly available commercial tests.6 DST may provide the only way of identifying resistant strains in such a scenario, However the time to get the result is prolonged.
Consolidated guidelines for the management of drug-resistant TB released by WHO and Programmatic Management of Drug Resistant Tuberculosis (PMDT) in India place emphasis on the use of “short course regimen,” “shorter oral bedaquiline-containing regimen,” and “all oral longer MDR TB regimen” [Table 2].7 For the management of DR TB, drugs have been categorized into three groups A, B, and C [Table 3].
| Types of tuberculosis regimen | ||
|---|---|---|
| Drug sensitive TB | Intensive phase –RHEZ (2 month) | Continuation phase – RHE (4 months) |
| H mono/poly DR-TB | (6/9) Lfx RZE | |
| MDR/RR TB | Regimen | |
| Shorter all-oral bedaquiline-containing regimen | (4–6) Bdq (6) Lfx Ciz Z E Hh Eto | (5) Mfxh Cfz Z E |
| All oral longer MDR TB regimen | (18–20) Bdq (6) Lfx Lzd Cfz Cs |
H: Isoniazid, Hb: High-dose isoniazid, R: Rifampicin, Z: Pyrazinamide, E: Ethambutol, Lfx: Levofloxacin, Mfxh: High-dose moxifloxacin, km: Kanamycin, Am: Amikacin, Eto: Etoposide, Cfz: Clofazimine, Bdq: Bedaquiline, Cs: Cycloserine, Lzd: Linezolid, DR-TB: Drug resistant tuberculosis, RR-TB: Rifampicin resistant tuberculosis, MDR-TB: Multidrug-resistant tuberculosis.
| Group A | Group B | Group C |
|---|---|---|
|
Levofloxacin (Lfx) or moxifloxacin (Mfx) Bedaquiline (Bdq) Linezolid (Lzd) |
Clofazimine (Cfz) Cycloserine (Cs) or Terizidone (Trd) |
Ethambutol (E) Delamanid (Dlm) Pyrazinamide (Z) Imipenemcilastatin (IpmCls) or meropenem (Mpm) Amikacin (Am) or Streptomycin (S) Ethionamide (Eto) or Prothionamide (Pto) Paraaminosalicylic acid |
The burden of MDR-TB is ever increasing and patients with cutaneous tuberculosis are not untouched by this, and it is probably on the rise too. Being pauci bacillary, these MDR strains tend to evade detection, and as such universal culture and DST should be done so as to treat the index cases and prevent further spread of primary DR-TB. The newer rapid tests, though giving quick results, have their limitations. Whole-genome sequencing appears to be the answer to the current problems, and if we are to address the situation effectively, the medical infrastructure needs to be strengthened sooner than later to identify the DR-TB strains rapidly and comprehensively.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Global tuberculosis report. Available from: http://www.who.int/tb/publications/global_report/en/2022. [Last accessed on 2022 Feb 07]
- Prevalence and risk factors of drug-resistant extrapulmonary tuberculosis. Clin Respir J. 2018;12:2101-9.
- [CrossRef] [PubMed] [Google Scholar]
- Report of the first national anti-tuberculosis drug resistance survey: India 2014–16. https://tbcindia.gov.in/showfile.php?lid=3315
- Cutaneous tuberculosis: Epidemiological, clinical, diagnostic and therapeutic update. Anais Brasileiros de Dermatologia. 2022;97:129-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for multidrug-resistant tuberculosis: A worldwide systematic review and meta-analysis. PLoS ONE. 2022;17:e0270003.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mycobacterium tuberculosis whole genome sequences from southern India suggest novel resistance mechanisms and the need for region-specific diagnostics. Clin Infect Dis. 2017;64:1494-501.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO consolidated guidelines on tuberculosis, module 4: treatment drug resistant tuberculosis treatment. Available from: https://www.who.int/publications/i/item/9789240007048. [Last accessed on 2022 Feb 07]






