Translate this page into:
Profile of Indian patients with premature canities
2 Department of Neurochemistry, Institute of Human Behavior and Allied Sciences, New Delhi, India
Correspondence Address:
Archana Singal
Department of Dermatology and STD, University College of Medical Sciences and GTB Hospital, University of Delhi, New Delhi - 110 095
India
| How to cite this article: Daulatabad D, Singal A, Grover C, Chhillar N. Profile of Indian patients with premature canities. Indian J Dermatol Venereol Leprol 2016;82:169-172 |
Abstract
Background: Premature canities is a common yet incompletely understood dermatological entity with scarce demographic and clinical data. Aim: Evaluation of the demographic and clinical profile of cases with premature canities and to look for systemic associations. Methods: Fifty two self-reported cases of premature canities (onset before 20 years of age) and an equal number of healthy controls were recruited from the outpatient department of the Department of Dermatology, Guru Teg Bahadur Hospital Delhi, India from November 2011 to March 2013. A detailed history including onset, duration and pattern of involvement, a family history with pedigree charting and scalp examination were recorded on a predesigned proforma. A history of atopy was looked for in all study subjects and they were screened for thyroid disorder and diabetes. Results: The mean age of cases and controls was comparable. The mean age of onset of graying was 11.6 ± 3.6 years. The mean duration at the time of presentation was 39.8 ± 37.2 months. The frontal region was the earliest affected area in 25 (48.1%) cases. Positive family history of premature canities was reported in 39 (75%) cases with an equal prevalence on paternal and maternal sides. More than half of the cases, 29 (55.8%) reported having a first degree relative affected by premature canities, 13 (25%) had a second degree and 20 (38.5%) had a third degree relative affected. Atopy was found to be strongly associated with premature canities with an odds ratio of 3.8. No association with thyroid abnormality or diabetes mellitus was seen. Limitation: The study suffered from the limitation of a small sample size. Conclusion: It was observed that the process of graying mostly starts in the frontal region. It was also found to be associated with a strong family history and atopic predisposition. Larger studies are recommended to arrive at a definite conclusion.INTRODUCTION
Graying of hair, scientifically termed as "canities," is a physiological phenomenon and considered to be a part of chronological aging. The normal occurrence of physiological graying of hair in white races is at the age of 34.2 ± 9.6 years. [1] In black races, the onset is slightly later at 43.9 ± 10.3 years and in Japanese between 30 and 34 years in men and between 35 and 39 years in women. The hair in beard and moustache areas commonly gray earlier than scalp or body hair. [2] There is no available reference age range for graying for the Indian subcontinent. When graying begins before the usual age of onset, it is termed premature canities. Hair is said to gray prematurely if it occurs before the age of 20 years in Caucasians and before 30 years in Africans. [3],[4],[5] Definition of premature canities with respect to the Asian population is lacking. However, a cut-off at the age of 25 years was once considered from India. [6]
Of late a re-emergence of interest has been observed in this seemingly mundane disorder by researchers and clinicians alike. It assumes a special significance in the Indian population in view of the dark hair color wherein even a few gray strands are easily noticeable. The present study was carried out to describe the clinical profile of premature canities in Indian patients.
METHODS
The study was conducted in the outpatient department of dermatology, University College of Medical Sciences and associated Guru Teg Bahadur Hospital, Delhi, India from November 2011 to March 2013. As the onset of premature canities has not been defined in our population, we selected a cut-off age of 20 years for the purpose of recruiting patients; the rationale being that the study population was North Indian (largely derived from the Anglo-Indian races) and could use the same cut-off of 20 years as used by Caucasian race. Institutional ethical committee approved the study protocol.
Patients with graying of hair as a part of other conditions such as vitiligo, cutaneous disease involving the scalp, history of topical application or systemic drug/supplement intake and use of hair dye in the past 6 months were excluded. Smokers, pregnant or lactating women and subjects with a history of any febrile illness or known systemic disease within 3 months of the onset of graying of hair were also excluded.
A detailed history the age of onset, pattern, progression and family history of graying were recorded on a predesigned proforma. Scalp examination was done for all cases by dividing the scalp surface into five zones, that is, frontal region, vertex, right and left temporal regions and the occipital region. In each zone, an area showing the maximum density of gray hair was identified. Within this area, a 1 cm 2 area was marked for trimming the hair and imaging. The images were displayed on a computer screen at high magnification. The density of white hair was calculated after manually counting the number of white and black hair seen in the pre-marked square area on screen.
A family history of premature graying was elicited and pedigree charting of three generations was also recorded based on recall. Patients were questioned regarding the history of atopy, thyroid disorder or diabetes and all cases were screened by estimation of fasting and postprandial blood sugars and thyroid function tests.
RESULTS
Demographic profile
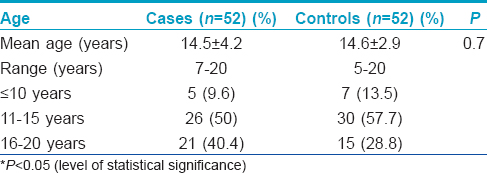
The study included 52 cases with premature onset of graying of hair (before the age of 20 years) and an equal number of age and sex matched controls. Of 52 cases, 27 (51.9%) were women and 25 (48.1%) were men [Table - 1]. The control group had a similar population of women and men (27 and 25, respectively). The mean age of cases and controls was also comparable.
Clinical profile
Age at onset
The mean age at onset of graying was 11.6 ± 3.6 years (range 3-18 years). The earliest age of onset recorded was 3 years reported by a 10-year-old boy. The majority of cases (n = 46; 88.5%) reported onset of graying before 16 years of age.
Duration and progression of graying
The mean duration of graying at the time of presentation was 39.8 ± 37.3 months (range: 4 months-15 years). Although 26 (50%) cases reported rapid progression of graying, the remaining reported slow progression. The difference in the duration of graying in both these groups was statistically significant (27.7 vs. 51.9, P = 0.02).
Origin of graying
Though at the time of presentation all cases had diffuse graying of hair, the onset of graying was reported from a specific region of the scalp. The frontal region was noticed to be the first affected area by 25 (48.1%) cases followed by the vertex in 18 (34.6%), occiput in 7 (13.5%) and temporal region in 2 (3.8%). The frontal and vertex areas were also the most affected regions. Other hairy sites such as eyelashes, beard, moustache, sideburns and axillae or groins were not involved in any of the study subjects.
Pedigree charting
A positive family history of premature canities was obtained in 39 (75%) of the cases. The three generation pedigree charting revealed a total of 96 relatives of 52 cases with premature graying. Of these relatives, 51 (53.1%) were men and 45 (46.9%) were women. Hence, no obvious sexual predilection was observed. Paternal and maternal relatives were equally affected with 13 (25%) cases each reporting positive history in maternal or paternal relatives, 5 (9.6%) cases having a concomitant family history in both paternal, as well as maternal relatives and 8 (15.4%) cases had a first degree relative affected in isolation. First degree relatives (siblings and parents) were to most commonly affected ([Table - 2]).

Atopic diathesis
History of atopic diathesis could be elicited in a significantly higher proportion of cases as compared to controls (19 [36.5%] vs. 5 [9.6%]; odds ratio of 3.8).
The mean blood glucose levels (86.6 ± 9.9 mg/dl in cases vs. 86.2 ± 7.8 mg/dl in controls; P = 0.85) thyroid stimulating hormone, free T 3 and T 4 were all within normal limits and comparable in both cases and controls.
DISCUSSION
Graying of hair is a physiological phenomenon and by the age of 50 years, 50% of the population have at least 50% gray hair known as the 50/50/50 rule of thumb. [1] The validity of this rule of thumb has been questioned by a large population-based study which reported 6-23% of people to have 50% gray hair by 50 years of age. [7]
Hair color is derived from the hair follicle pigmentary unit which functions maximally during post-adolescence and early adulthood. [8] Graying of hair is believed to have a multifactorial etiology which includes a genetic component, environmental factors, nutritional status and oxidative stress but the exact cause or pathogenesis has not been detected. It is considered that photodamage which has a major role in skin aging does not to hasten graying of hair. [9]
In our study the mean age of seeking medical attention was around adolescence, that is, 14.5 ± 4.3 years with no obvious sex preponderance, similar to that documented by Bhat et al.[10] This age group is very conscious of their self-image and generally governed by peer-pressure and graying can lead to tremendous psycho-social impact. The age of onset of premature canities cannot be compared with any other study as this parameter has not been evaluated previously.
The duration of graying at the time of presentation showed a marked variation from 4 months to 15 years, suggestive of a marked variation in time to seek medical attention. This may be due to the benign nature of the problem and use of household remedies. The progression of graying was categorized as rapid or slow based on the individual perception of the patient. Subjects with slow progression took almost double the time (mean duration: 51.9 ± 44 months) to seek medical advice when compared with those who had rapid progression (mean duration: 27.7 ± 24.3 months).
All cases presented with diffuse graying that began from a specific region of the scalp with no tendency to involve the scalp margins. The majority of the cases noticed the first strands of gray hair in the frontal and vertex region of the scalp, this could be because these sites are most easily amenable to examination. None of the cases had involvement of the sideburns or hair other than the scalp hair. This is in sharp contrast to the pattern of physiological graying which starts from the temporal region and sideburns in men and scalp margins in women. This prompts us to hypothesize that premature canities could be a distinct entity by itself; and not just be a part of chronological graying, although it may merge with the latter at a later age. In a study from Korea, it was reported that the parietal or occipital area was first involved in cases with early onset of graying (onset before 40 years of age) whereas the frontal area was involved in those with late onset of graying (after 40 years). [11] The difference could reflect a racial variation or may also be a result of a much higher cut-off being designated by the investigators. Earlier reports have indicated a possible autosomal dominant inheritance for premature canities, though it has not been proven; our study indicates a similar pattern. [3] However, we acknowledge that elaborate pedigree charting on a larger sample size is required to establish the mode of inheritance. As mentioned earlier, the first degree relatives were found to be maximally affected followed by the third and then the second degree relatives, suggesting a complex interplay of genetic and environmental factors. Though genetic factors appear to be crucial, the higher percentage of involvement of the third degree relative when compared with the second degree suggests a strong role of an environmental factor in addition to genetic predisposition. The unavoidable increase in the level of environmental pollution, stressful lifestyle and drastic change in the dietary habits in the past few decades may contribute to relatively early age of the onset of premature canities especially in genetically predisposed people. An association with a positive family history has also been recently suggested by a questionnaire-based study in men younger than 30 years of age. [12]
In the present study, cases with premature canities had a nearly 4-fold risk of atopy as compared to controls. However, it is not known whether atopy plays any role in its etiopathogenesis. Furthermore, no association with diabetes or thyroid disorders was noted. Studies with larger sample size are recommended to draw a definite conclusion.
Limitations
A small sample size is a major limitation of this study. Additionally the study population may not be truly representative of the general population as this was a hospital based study and only cases who sought medical care were recruited. Although the method of scalp examination followed can help quantify the pattern and extent of graying, but it is tedious and time-consuming and may not be feasible in a busy outpatient setup.
CONCLUSION
The present study reveals that the pattern of graying in premature canities is distinctly different from that of physiological graying. A suggested cut-off age of 20 years may be useful for identifying patients with premature canities for medical and academic purposes. Multiple etiologic factors seem to be at play with atopy and family history showing multiple possible causative mechanisms which need to be explored further. This preliminary study provides demographic data from North India which can be further evaluated in large scale studies with larger number of patients involving more geographical and ethnic backgrounds.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Keogh EV, Walsh RJ. Rate of greying of human hair. Nature 1965;207:877-8.
[Google Scholar]
|
| 2. |
Messenger AG, de Berker DA, Sinclair RD. Disorders of hair. In: Breathnach S, Cox N, Griffiths C, Burns T, editors. Rook′s Textbook of Dermatology. 8 th ed. New Jersey: Wiley-Blackwell; 2010. p. 66.1-66.100.
[Google Scholar]
|
| 3. |
Trüeb RM. Pharmacologic interventions in aging hair. Clin Interv Aging 2006;1:121-9.
[Google Scholar]
|
| 4. |
Odom RB, James WD, Berger TG, editors. Diseases of the skin appendages. In: Andrew′s Diseases of the Skin Clinical Dermatology. 9 th ed. Philadelphia: WS Saunders; 2000. p. 955.
[Google Scholar]
|
| 5. |
Tobin DJ, Paus R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp Gerontol 2001;36:29-54.
[Google Scholar]
|
| 6. |
Pasricha JS, Verma K. Diseases of the appendages. In: Treatment of Skin Diseases. 5 th ed. New Delhi: Mehta Publishers; 2008. p. 289.
[Google Scholar]
|
| 7. |
Panhard S, Lozano I, Loussouarn G. Greying of the human hair: A worldwide survey, revisiting the ′50′ rule of thumb. Br J Dermatol 2012;167:865-73.
[Google Scholar]
|
| 8. |
Pandhi D, Khanna D. Premature graying of hair. Indian J Dermatol Venereol Leprol 2013;79:641-53.
[Google Scholar]
|
| 9. |
Tobin DJ. Aging of the hair follicle pigmentation system. Int J Trichology 2009;1:83-93.
[Google Scholar]
|
| 10. |
Bhat RM, Sharma R, Pinto AC, Dandekeri S, Martis J. Epidemiological and investigative study of premature graying of hair in higher secondary and pre-university school children. Int J Trichology 2013;5:17-21.
[Google Scholar]
|
| 11. |
Jo SJ, Paik SH, Choi JW, Lee JH, Cho S, Kim KH, et al. Hair graying pattern depends on gender, onset age and smoking habits. Acta Derm Venereol 2012;92:160-1.
[Google Scholar]
|
| 12. |
Shin H, Ryu HH, Yoon J, Jo S, Jang S, Choi M, et al. Association of premature hair graying with family history, smoking, and obesity: A cross-sectional study. J Am Acad Dermatol 2015;72:321-7.
[Google Scholar]
|
Fulltext Views
7,422
PDF downloads
2,635





