Translate this page into:
Psoriasis in India: Prevalence and pattern
Correspondence Address:
Sunil Dogra
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: Dogra S, Yadav S. Psoriasis in India: Prevalence and pattern. Indian J Dermatol Venereol Leprol 2010;76:595-601 |
Psoriasis is an immune mediated genetically determined common dermatological disorder which affects skin, nails, joints and has various systemic associations. There is evidence that the disease is associated with a high impact on the health-related quality of life and considerable cost. Large literature has been published focusing on its varied aspects. There is a no dearth of information available; however, many questions remain that are still unanswered. This review reflects basic epidemiological data on Indian psoriasis patients to provide a comprehensive overview of psoriasis in India.
Prevalence
There is a growing number of population-based studies providing worldwide prevalence estimates of psoriasis. Prevalence of psoriasis varies in different parts of the world. According to published reports, prevalence in different populations varies from 0% to 11.8%. [1],[2],[3],[4] For most of the data given, the range extends from around 0.5% to close to 2.5%. In the USA, the prevalence of psoriasis was estimated to be around 4.6% while in Canada it was 4.7%. Data from Europe show little variation in countries with a range from 1.4% (Norway), 1.55% (Croatia) and 1.6% (UK). In East Africa, the figure was 0.7% and in the Henan district of China only 0.7% were found affected. [5]
Most of the data on prevalence has been derived from hospital-based studies while there are only few well-defined large population based studies done to find the exact prevalence of this dermatoses in the community. [6],[7],[8],[9]
Prevalence studies from India are mostly hospital-based. [Table - 1] presents the comparative data from various epidemiological studies on psoriasis from India. Okhandiar et al. [10] collected a comprehensive data from various medical colleges located in Dibrugarh, Calcutta, Patna, Darbhanga, Lucknow, New Delhi and Amritsar. They found that the incidence of psoriasis among total skin patients ranged between 0.44 and 2.2%, with overall incidence of 1.02%. They noted that the incidence in Amritsar (2.2%) was higher as compared to other centers in Eastern India and speculated that it may be related to different environmental conditions (extremes of temperature), dietary habits, and genetic differences. The ratio of male to female (2.46:1) was very high which could not be clearly accounted for. Highest incidence was noted in the age group of 20-39 years and the mean age of onset in males and females was comparable.
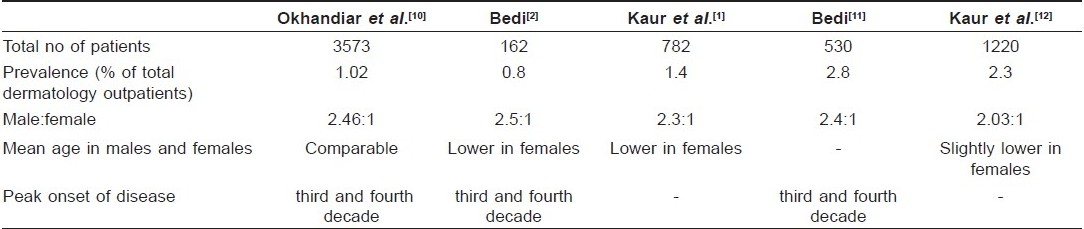
In another study from North India, Bedi [2] reported the prevalence of psoriasis to be 0.8% among the skin patients but the sample size of the study was very small. Male to female sex ratio was 2.5:1. In this study, it was observed that females had lower mean age of onset compared to males. In a latter study by Bedi, [11] which included larger number (530) of subjects, prevalence of psoriasis among dermatology outpatients was found to be 2.8% while male to female ratio continued to be the same.
In a study from tertiary health care center from North India, psoriasis patients accounted for 2.3% of the total dermatology outpatients. [12] Of the total psoriasis patients, 67% were men and 33% were women, male to female ratio being 2.03:1. Ages of patients ranged from infancy to eighth decade, mean age being 33.6 years. Children accounted for 4.4% of total psoriasis patients. Women had slightly lower mean age of onset (27.6 years) compared to the men (30.9 years).
So, it can be inferred that in India the prevalence of psoriasis varies from 0.44 to 2.8%, it is twice more common in males compared to females, and most of the patients are in their third or fourth decade at the time of presentation. These studies are limited by the absence of commonly accepted and validated diagnostic criteria. Furthermore, there is basically no reliable information on time trends of the disease.
Genetics and Familial Incidence
Psoriasis vulgaris is known to be associated with certain HLA antigens and complement factors but most of the studies published are in Western populations with only little information about Indians. Chablani et al. [13] in a study of 67 psoriasis subjects from Western India found association with the A1, B17 and Cw6, but not with B13 antigens. Pitchappan et al. [14] reported association of HLA Bw57 and DR7 with psoriasis vulgaris in South India. Rani et al. [15] showed that Cw FNx010602 was the main allele that had high frequency in psoriasis patients in North India.
Genetic predisposition has a significant role in the etiopathogenesis of psoriasis and familial clustering of the cases has been observed. Farber et al. [9] reported familial occurrence in 36% of their patients. Familial incidence is greater in childhood psoriasis compared to adult onset psoriasis. [16],[17],[18]
Indian studies report lower familial incidence of the disease. Bedi [11] reported positive family history of psoriasis in 14% of their patients. While Kaur et al. [12] reported family history in only 2% of their patients. First degree relatives were affected in 84% of the cases while second degree relatives in 12% cases. [12] There are only few studies which have made record of family history of psoriasis in their patients, so definite statistical data on familial incidence is not available.
Clinical Pattern
There are varied clinical presentations of psoriasis. Broadly, it has been classified into non pustular and pustular psoriasis. There are further clinical phenotypes of each. It is important to recognize precisely the clinical subtype as it has implications on the choice of treatment modality and also reflects upon the disease activity. Most common clinical type is the classic chronic plaque type psoriasis. But even same clinical subtype can behave differently in different individuals.
Henseler and Christopher [19] on the basis of phenotype database of 2147 patients, recognized two distinct patient cohorts. One cohort had early onset (type I) of psoriasis in the second decade and the other cohort had late onset (type II) of the disease in the fifth decade. Early onset cohort had more widespread and recurrent disease, and also higher number had affected parents compared to late onset cohort.
There are only few studies from India which have analyzed the clinical spectrum of the disease in psoriasis patients [Table - 1]. Okhandiar et al. [10] collected epidemiological data of 116 psoriasis patients from various medical colleges. They found that the extensors (93%) were the most common site of involvement followed by the scalp (88%). Face, palm, sole and nail were affected in one third of the cases. Inverse psoriasis was uncommon. None of their patient had mucosal involvement and also they did not comment on the morphological types of psoriasis in their patients.
Bedi [11] analyzed data of 530 psoriasis patients seen over a period of five years. Chronic plaque type psoriasis was the most common (90%) clinical phenotype. The most common sites of involvement in descending order of frequency were trunk, limbs, scalp, face, palms-soles and flexures. The second most common clinical phenotype was palmoplantar psoriasis followed by flexural psoriasis. He found guttate psoriasis, mucosal involvement and psoriatic erythroderma to be uncommon.
Kaur et al. [12] reported scalp (25%) as the most common first site of involvement followed by legs (20.6%) and arms (11.7%). Oral (0.7%) and genital (0.4%) mucosal involvement was found to be uncommon. Chronic plaque type psoriasis (93%) was the most common clinical phenotype. Palmoplantar pustulosis, guttate and erythrodermic variants accounted for less than 2% of cases each. Generalized pustular, isolated nail, flexural and arthropathic forms were very uncommon.
Pattern of post-inflammatory pigmentation following clearance of lesions was reported by Kaur et al. [12] They noted that psoriasis lesions cleared leaving no dyspigmentation in majority of the cases, while hyperpigmentation and hypopigmentation followed clearance of lesions in 9.7% and 7.3% of the patients, respectively.
Two studies by Kaur et al. [1],[12] have presented the data on severity of disease in their patients. In the initial study which included 782 patients, the extent of disease was 50% body surface area (BSA) involvement in two-third of patients, 50-70% BSA involvement in 15% and less in the rest. Recent study comprising of 1220 psoriasis patients reported, <25% BSA involvement in three-fourth patients, one fifth had 26-50%, and <10% had >50% BSA involvement. [12] There are no Indian studies to provide data on severity of disease among psoriasis patients based on PASI scores.
Besides being cosmetically disabling, psoriasis leads to morbidity due to pruritus and, burning sensation in many patients. Okhandiar et al. [10] found pruritus to be present in 95% of their cases, while burning and smarting sensation in around 15% cases. Bedi [2] noted itching to be a significant complaint in 81% of the patients. Kaur et al. [12] found that burning and stiffness were uncommon (1%) while pruritus was complained by 65% patients. So, the results of various studies suggest that psoriasis is definitely a pruriginous disorder adding to the morbidity of this dermatosis.
Relapses are common and the patterns of relapses are also varied in different individuals. Some have early and frequent relapses and others have long-term remissions of their disease process with infrequent relapses. There is scarcity of data on the relapse pattern of psoriasis from India. Kaur et al. [12] reported that the duration of remissions varied widely from two weeks to nine years in their patients but 4% of their patients never had complete remission of their disease.
Bedi [2] noted seasonal variation in 46% patients, half of whom felt worsening in winters. Similarly, Kaur et al. [12] reported seasonal variation in half of their patients, with 43% reporting improvement in summer and 7% in winter.
Erythrodermic psoriasis
Psoriasis is an important and common cause of erythroderma. Psoriatic erythroderma can appear de novo or it occurs when chronic plaque type psoriasis leads to extensive involvement. Sarkar et al.[20] studied 17 cases of childhood erythroderma over a period of five years suggesting that psoriatic erythroderma is uncommon in children (18% of the total cases)compared to the adults. In a recent study by Prakash et al.,[21] psoriasis was clinically suspected in 12 of the total 24 patients of erythroderma. Nanda et al.[22] observed erythroderma in 2 (1.8%) out of 112 children with psoriasis seen over nine years (1980 to 1988) at North India.
Nail psoriasis
Nail involvement is common in psoriasis and can be the initial and the only site of involvement in some patients. Morphology of nail changes depends on whether nail matrix, nail bed or hyponychium has been affected. Nail involvement is more common in those who have concurrent psoriatic arthritis. [23] Bedi [2] noted nail changes in 74% of total patients while isolated nail affliction was seen in 6% of cases. Various nail changes observed in descending order of frequency were pitting, thickening of nail plate, partial onycholysis, subungual hyperkeratosis, yellow-brown discoloration, paronychia, and complete onycholysis.
Kaur et al. [24] studied nail changes in 167 psoriasis patients over a period of five years. Of the total cases, 3% had isolated nail involvement. Pitting was the most common nail change, followed by onycholysis, discoloration, subungual hyperkeratosis, longitudinal ridging and thickening of the nail plate. Pits were present in irregular pattern in 90% cases while in rest 10% they were arranged in transverse or longitudinal lines. Two third of their subjects did not have nail fold involvement. While one third cases who had proximal and /or lateral nail fold involvement, had larger body surface area involved and also had significantly higher mean number of nail changes per subject.
Ghosal et al.[25] studied nail involvement in 100 psoriasis patients. Finger nails were involved in 32% and toe nails in 24% cases. Pitting and subungual hyperkeratosis were the most common finger and toe nail changes noted in 65% and 33% cases, respectively. Nail changes were more common in those with joint pain (73%) compared to those who had no joint complaints (30%) and odds ratio was 6.6. They also noted significant association of nail changes with Koebner phenomenon.
Palmoplantar psoriasis
Palmoplantar involvement can occur in isolation but often occurs with psoriasis elsewhere on the body. Though only small body surface area is affected but because of the disabling sequalae, this site of involvement carries a definite significance. Palmoplantar involvement can occur in the form of scaly plaques or pustular lesions which can present as palmoplantar pustulosis or acrodermatitis continua of hallopeau.
Kumar et al.[26] have done a comprehensive study involving 540 patients having significant palmar and /or plantar involvement with or without psoriasis elsewhere over the body. Those having extensive psoriasis (>30 % BSA) or erythrodermic psoriasis or generalized pustular psoriasis were excluded from the study. Out of total cases, only 15 (2.8%) had pustular lesions, 11 had palmoplantar pustulosis and 4 had acrodermatitis continua of hallopeau. Males outnumbered females and the male to female ratio was similar to that observed in chronic plaque type psoriasis. Most of the patients had involvement of both palms and soles (47.5%) or palms alone (44.3%) while isolated sole (8.1%) involvement was uncommon. The most common pattern of involvement was of plaque type lesions situated over pressure bearing areas on palms and, discrete plaques present randomly over the soles. Extension of lesions onto dorsum of hand and feet and inter digital spaces was also observed. Palmoplantar psoriasis leads to morbidity due to itching, fissuring, difficulty in walking and performing manual tasks. Author concluded that friction plays a role in localizing the lesions over certain areas on palm and sole in patients with pre-existing palmoplantar psoriasis. High prevalence of plantar involvement was ascribed to Indian custom of walking bare foot or wearing open slippers most of the time which leads to frequent minor trauma compared to closed footwear.
Childhood psoriasis
Psoriasis is relatively uncommon but a burdensome disease in children. There are few epidemiological studies on childhood psoriasis. Infantile psoriasis is less defined as there is clinical overlap with other common dermatoses of this age group like atopic dermatitis and seborrhoeic dermatitis. Psoriasis in children has few peculiar features which have been consistently observed in studies on childhood psoriasis. [27],[28] Guttate psoriasis is more common, spontaneous remissions are relatively more common than in adults while relapses usually follow infections. [29] Certain clinical variants like erythroderma, localized and generalized pustular psoriasis and arthropathy are uncommon. [27],[28],[29],[30],[31]
Western literature reports psoriasis prevalence to be around 0.71% in children. [32] The only comprehensive prevalence analysis of juvenile psoriasis till date showed the prevalence in United Kingdom of about 0.6% in children aged 0-9 years and 1.4% in children aged 10-19 years. [33] In a large series of 1262 psoriasis children from Australia, age of patients ranged from 1 month to 15 years, there was equal incidence in male and female, family history was present in a significant number of cases (71%), plaque type of psoriasis was the most common clinical phenotype (34%), facial involvement was common (38%) and infantile psoriasis presented as diaper rash commonly. [34]
In a study from South India done to evaluate the pattern of dermatoses in children, psoriasis accounted for 1.4% of the total skin patients presenting in an out patient department of a hospital over a period of one year. [35] In India, largest series on childhood psoriasis was reported by Kumar et al. [36] They presented data of 419 children with psoriasis. Children accounted for 12.5% of total psoriasis patients over a period of 13 years. Age of onset ranged from 4 days to 14 years, male and female incidence was equal, and plaque type psoriasis was the most common clinical presentation. Unlike the study by Morris et al. [34] familial incidence was low (4.5%) and facial involvement was uncommon.
Associations
There are several associations of psoriasis including arthritis, immunobullous disorders, vitiligo, metabolic syndrome and synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome. Arthritis is the most common association first recognized by Jean Louis Alibert in 1818. In 1973, Moll and Wright [37] clearly defined psoriatic arthritis (PsA) as a distinct entity under seronegative spondyloarthropathies and gave simple criteria for its diagnosis which include; an inflammatory arthritis (peripheral arthritis and/or sacroiliitis or spondylitis); presence of psoriasis; absence (usually) of serological tests for rheumatoid factor. They also described its five main clinical patterns viz: distal interphalangeal (DIP) arthritis, arthritis mutilans (destructive), symmetric polyarthritis, asymmetric oligoarthritis and spondyloarthropathy. Other manifestations of PsA include enthesitis, tendonitis, fasciitis, and dactylitis.
The incidence of PsA is estimated to range from 7% to 42% among all psoriasis patients. [38] There are only few studies on PsA from India. Peak incidence of PsA has been reported in the fifth and sixth decade of life. [39],[40],[41] In most of the studies from India, males outnumbered females unlike rheumatoid arthritis (RA) which is more common in females. [39],[40],[41],[42],[43] But the literature from west suggests female preponderance. [23],[44] The most common pattern of PsA reported in Western literature is asymmetric oligoarticular [45],[46] However, Ray et al. [41] and Rajendran et al. [39] found polyarticular pattern simulating RA as the most common pattern. Isolated DIP arthritis is uncommon and arthritis mutilans is rare. [39] Psoriasis lesions precede the onset of PsA in more than 70% cases. [39],[40],[41],[42],[43] PsA usually appears within 10 years of the onset of psoriasis and the chances of development after 10 years are very low. . Significant number of psoriasis patients (27.5%) have positive family history. [40]
The most common clinical phenotype observed in PsA patients is the chronic plaque type which is otherwise also the most common clinical presentation. [39],[40],[41] Nail changes are common in psoriasis patients having concurrent PsA occurring in around 70% patients. [39],[40] The most common findings observed are pitting, subungual hyperkeratosis, discoloration, ridging and thickening of nail plate. [40],[41] HLA association has been studied in few studies. Ray et al. [41] found positive association of HLA-A1 and - B17 with the peripheral psoriatic arthritis.
Recently, the association of psoriasis with metabolic syndrome (MS) has gained considerable attention in literature. Large number of studies in Western literature suggests association of psoriasis with MS comprising obesity, heart disease, hyperglycemia, dyslipidemia and hypertension. [47],[48] It has been recognized to contribute to the mortality risk in this group of patients. [49],[50] There are no Indian studies to elucidate the role of MS in Indian patients. There is evidence for association of several lifestyle factors including alcohol and smoking and other diseases with psoriasis. But it can be reliably confirmed by appropriate study designs (cohort studies) which will help to elucidate the true associations and disentangle the temporal sequence.
Bullous pemphigoid and vitiligo are two autoimmune diseases reported to be associated with psoriasis. More than 50 cases have been reported in literature, with coexisting psoriasis and bullous pemphigoid. [51],[52] Vitiligo coexisting and co-localizing with psoriasis has been reported from India. [53],[54],[55] Dhar et al. [54] reported co-localization of vitiligo and psoriasis in a 9-year-old boy and concluded that, there could be structural similarities between anti stratum corneum antibodies and anti melanocyte antibodies and that a common neuropeptide might be responsible for co-habitation of vitiligo and psoriasis. Kaur et al. [12] reported vitiligo as the most common cutaneous disease (1.7%) found to be associated with psoriasis. In a retrospective study (1989-2003), Sandhu et al. [55] noted presence of vitiligo in 38 out of 4700 total psoriasis patients seen over this period. There was no definite pattern of distribution; psoriasis and vitiligo were distributed separately in 18 (47.3%) patients, whereas overlapping pattern where psoriasis lesions partly or completely covered some of the vitiliginous patches and also occurred at nonvitiliginous sites were seen in 13 (34.2%) patients. They did not find increased incidence of concurrence of both diseases, and the onset and course of the psoriasis and vitiligo were independent of each other. Psoriasis lesions occurred on vitiliginous areas and normal skin with equal frequency, with strict co-localization being an uncommon feature.
Psychiatric comorbidity
Psoriasis produces significant adverse effects on the psychological and social aspects of life mainly because of its visibility. [56],[57],[58] There are few studies from India addressing this aspect of the disease.
Rakhesh et al. [59] studied 50 psoriasis patients aged more than 18 years assessing their clinical severity, physical and psychosocial disability and stress incurred by measuring psoriasis area severity index (PASI), psoriasis disability index (PDI), and psoriasis life stress inventory (PLSI), respectively. Among the physical and psychosocial factors investigated, daily activities, employment, and treatment were most affected. They also concluded that psoriasis sufferers feel self-conscious, disturbed / inconvenienced by the shedding of the skin, live in a constant fear of relapse, and avoid social interactions.
Gaikwad et al. [60] in a study of 43 psoriasis patients found that their disease affected the social functioning, led to decreased work efficiency and subjective distress at work in more than half of the subjects. Around two third patients consented that psoriasis has affected their interpersonal relationship resulting in stress in home environment. Sixty-seven percentage of the patients had psychiatric comorbidity. Mattoo et al. [61] studied psychiatric morbidity in 103 psoriasis patients and 113 vitiligo patients at hospital settings in Chandigarh. The general health questionnaire (GHQ) assessed psychiatric morbidity rates at 33.63% and 24.7% for vitiligo and psoriasis, respectively. The ICD-10 psychiatric diagnoses in GHQ positive cases were adjustment disorder (56% vs 62%), depressive episode (22% vs 29%) and dysthymia (9% vs 4%) in vitiligo and psoriasis, respectively. The comprehensive psychopathological rating scale (CPRS) assessed that depression, anxiety, and total psychopathology levels were similar in the two GHQ positive subgroups.
So, these studies suggest that psoriasis leads to significant psychosocial disability in the patients and this facet of the disease should be addressed while managing any patient by means of psychological and behavior therapy.
Conclusion
Psoriasis is a common dermatological disorder in India with prevalence and epidemiological characteristics similar to the presentation of disease in West. There is paucity of data related to Indian psoriasis patients on its genetics, epidemiology, pustular psoriasis, childhood psoriasis, disease severity, relapse, remission patterns and its associations. More research and detailed prospective studies need to be done to delineate the natural course of the disease which varies in different individuals and also according to the clinical pattern of the disease. Prevention of psoriasis has barely been studied. A prerequisite would be that risk factors are identified in a consistent and reliable way. Primary and secondary prevention, however, is crucial in common chronic diseases where causative treatments are still lacking.
| 1. |
Kaur I, Kumar B, Sharma VK, Kaur S. Epidemiology of psoriasis in a clinic from north India. Indian J Dermatol Venereol Leprol 1986;52:208-12.
[Google Scholar]
|
| 2. |
Bedi TR. Psoriasis in north India. Geographical variations. Dermatologica 1977;155:310-4.
[Google Scholar]
|
| 3. |
Swanbeck G, Inerot A, Martinsson T, Wahlstrφm J. A population genetic study of psoriasis. Br J Dermatol 1994;131:32-9.
[Google Scholar]
|
| 4. |
Farber EM, Nall L. Epidemiology: natural history and genetics. In: Roenigk Jr HH, Maibach HI, editors. Psoriasis. New York: Dekker; 1998. p. 107-57.
[Google Scholar]
|
| 5. |
Christophers E. Psoriasis - epidemiology and clinical spectrum. Clin Exp Dermatol 2001;26:314-320.
[Google Scholar]
|
| 6. |
Lomholt G. Prevalence of skin diseases in a population: a census study from the Faroe Islands. Dan Med Bull 1964;11:1-7.
[Google Scholar]
|
| 7. |
Hellgren L. Psoriasis: The prevalence in sex, age and occupational groups in total populations in Sweden. Morphology, inheritance and association with other skin and rheumatic diseases. Stockholm: Almquist and Wiksell; 1967.
[Google Scholar]
|
| 8. |
Brandrup F, Green A. The prevalence of psoriasis in Denmark. Acta Derm Venereol 1981;61:344-6.
[Google Scholar]
|
| 9. |
Farber EM, Nall L. The Natural history of psoriasis in 5,600 patients. Dermatologica 1974;148:1-18.
[Google Scholar]
|
| 10. |
Okhandiar RP, Banerjee BN. Psoriasis in the tropics: An epidemiological survey. J Indian Med Assoc 1963;41:550-6.
[Google Scholar]
|
| 11. |
Bedi TR. Clinical profile of psoriasis in North India. Indian J Dermatol Venereol Leprol 1995;61:202-5.
[Google Scholar]
|
| 12. |
Kaur I, Handa S, Kumar B. Natural history of psoriasis: a study from the Indian subcontinent. J Dermatol 1997;24:230-4.
[Google Scholar]
|
| 13. |
Chablani UA, Contractor NM, Gadgil RB. HLA and complement C4 studies in psoriasis vulgaris. Natl Med J India 1992;5:8-11.
[Google Scholar]
|
| 14. |
Pitchappan RM, Koteeswaran A, Kakkaniah VN, Manickasundari M, Rajaram V, Muthuveeralakshmi P, et al. HLA Bw57 and DR7 association with psoriasis vulgaris in south India. Tissue Antigens 1989;34:133-7.
[Google Scholar]
|
| 15. |
Rani R, Narayan R, Fernandez-Vina MA, Stastny P. Role of HLA-B and C alleles in development of psoriasis in patients from North India. Tissue Antigens 1998;51:618-22.
[Google Scholar]
|
| 16. |
Farber EM, Jacobs AH. Infantile psoriasis. Am J Dis Child 1977;131:1266-9.
[Google Scholar]
|
| 17. |
Farber EM, Carlsen RA. Psoriasis in childhood. Calif Med 1966;105:415-20.
[Google Scholar]
|
| 18. |
Hansen AG. Psoriasis in childhood. In: Farber EM, Cox AJ, editors. Psoriasis: Proceedings of the International Symposium. Stanford, CA: Stanford University Press, 1971. p. 53-9.
[Google Scholar]
|
| 19. |
Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 1985;13:450-6.
[Google Scholar]
|
| 20. |
Sarkar R, Sharma RC, Koranne RV, Sardana K. Erythroderma in children: A clinico-etiological study. J Dermatol 1999;26:507-11.
[Google Scholar]
|
| 21. |
Prakash BV, Sirisha NL, Satyanarayana VV, Sridevi L, Ramachandra BV. Aetiopathological and clinical study of erythroderma. J Indian Med Assoc 2009;107:102-3.
[Google Scholar]
|
| 22. |
Nanda A, Kaur S, Kaur I, Kumar B. Childhood psoriasis: An epidemiologic survey of 112 patients. Pediatr Dermatol 1990;7:19-21.
[Google Scholar]
|
| 23. |
Scarpa R, Oriente P, Pucino A, Torella M, Vignone L, Riccio A, et al. Psoriatic arthritis in psoriatic patients. Br J Rheumatol 1984;23:246-50.
[Google Scholar]
|
| 24. |
Kaur I, Saraswat A, Kumar B. Nail changes in psoriasis: a study of 167 patients. Int J Dermatol 2001;40:601-3.
[Google Scholar]
|
| 25. |
Ghosal A, Gangopadhyay D, Chanda M, Das N. Study of nail changes in psoriasis. Indian J Dermatol 2004;49:18-21.
[Google Scholar]
|
| 26. |
Kumar B, Saraswat A, Kaur I. Palmoplantar lesions in psoriasis: A study of 3065 patients. Acta Derm Venereol 2002;82:192-5.
[Google Scholar]
|
| 27. |
Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol 2000;17:174-8.
[Google Scholar]
|
| 28. |
Farber EM, Mullen RH, Jacobs AH, Nall L. Infantile psoriasis: a follow up study. Pediatr Dermatol 1986;3:237-43.
[Google Scholar]
|
| 29. |
Nyfors A. Psoriasis in children. Characteristics, prognosis and therapy: A review. Acta Derm Venereol (Stockh) 1981;95:47-53.
[Google Scholar]
|
| 30. |
Hutton KP, Orenberg EK, Jacobs AH. Childhood psoriasis. Cutis 1987;37:26-7.
[Google Scholar]
|
| 31. |
Watson W, Farber EM. Psoriasis in childhood. Pediatr Clin North Am 1977;39:26-7.
[Google Scholar]
|
| 32. |
Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schδfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol 2010;162:633-6.
[Google Scholar]
|
| 33. |
Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: A population-based study. Arch Dermatol 2005;141:1537-41.
[Google Scholar]
|
| 34. |
Morris A, Rogers M, Fischer G, Williams K. Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol 2001;18:188-98.
[Google Scholar]
|
| 35. |
Karthikeyan K, Thappa DM, Jeevankumar B. Pattern of pediatric dermatoses in a referral center in South India. Indian Pediatr 2004;41:373-7.
[Google Scholar]
|
| 36. |
Kumar B, Jain R, Sandhu K, Kaur I, Handa S. Epidemiology of childhood psoriasis: a study of 419 patients from northern India. Int J Dermatol 2004;43:654-8.
[Google Scholar]
|
| 37. |
Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55-78.
[Google Scholar]
|
| 38. |
Gladman DD, Rahman P. Psoriatic arthritis. In: Ruddy S, Harris ED, Sledge CB, editors. Kelly's textbook of Rheumatology. 6 th ed. Vol 2. Philadelphia W.B Saunders Company; 2001. p. 1071-9.
th ed. Vol 2. Philadelphia W.B Saunders Company; 2001. p. 1071-9.'>[Google Scholar]
|
| 39. |
Rajendran CP, Ledge SG, Rani KP, Madhavan R. Psoriatic arthritis. J Assoc Physicians India 2003;51:1065-8.
[Google Scholar]
|
| 40. |
Prasad PV, Bikku B, Kaviarasan PK, Senthilnathan A. A clinical study of psoriatic arthropathy. Indian J Dermatol Venereol Leprol 2007;73:166-70.
[Google Scholar]
|
| 41. |
Ray SPC, Singh T, Kaur I, Suri S, Sehgal S, Kaur S. Clinical profile of psoriatic arthropathy. Indian J Dermatol Venereol Leprol 1990;56:200-3.
[Google Scholar]
|
| 42. |
Shah NM, Mangat G, Balakrishnan C, Joshi VR. Psoriatic arthritis - a study of 102 patients. J Indian Rheumat Assoc 1995;3:133-6.
[Google Scholar]
|
| 43. |
Nadkar MY, Kalgikar A, Samant RS, Borges NE. Clinical profile of psoriatic arthritis. J Indian Rheumat Assoc 2000;8:S40.
[Google Scholar]
|
| 44. |
Kononen M, Torppa J, Lassus A. An epidemiological survey of psoriasis in the Greater Helsiniki area. Acta Derm Venereol Suppl (Stockh) 1986;124:1-10.
[Google Scholar]
|
| 45. |
Kammer GM, Soter NA, Gibson DJ, Schur PH. Psoriatic arthritis: a clinical, immunologic and HLA study of 100 patients. Semin Arthritis Rheum 1979;9:75-97.
[Google Scholar]
|
| 46. |
Robert ME, Wright V, Hill AGS, Mehra AC. Psoriatic arthritis - follow up study. Ann Rheum Dis 1976;35:206-19.
[Google Scholar]
|
| 47. |
Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol 2007;157:68-73.
[Google Scholar]
|
| 48. |
Takahashi H, Takahashi I, Honma M, Ishida-Yamamoto A, Iizuka H. Prevalence of metabolic syndrome in Japanese psoriasis patients. J Dermatol Sci 2010;57:143-4.
[Google Scholar]
|
| 49. |
Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 2009;145:700-3.
[Google Scholar]
|
| 50. |
Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schδfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol 2010;162:633-6.
[Google Scholar]
|
| 51. |
Wilczek A, Sticherling M. Concomitant psoriasis and bullous pemphigoid: coincidence or pathogenic relationship? Int J Dermatol 2006;45:1353-7.
[Google Scholar]
|
| 52. |
Yasuda H, Tomita Y, Shibaki A, Hashimoto T. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology 2004;209:149-55.
[Google Scholar]
|
| 53. |
Inamadar AC, Sampagavi VV, Athanikar SB, Patil MN, Deshmukh NS. Vitiligo and psoriasis: coexistence with colocalization. Indian J Dermatol Venereol Leprol 2001;67:214-5.
[Google Scholar]
|
| 54. |
Dhar S, Malakar S, Dhar S. Colocalization of vitiligo and psoriasis in a 9-year old boy. Pediatr Dermatol 1999;1:242-3.
[Google Scholar]
|
| 55. |
Sandhu K, Kaur I, Kumar B. Psoriasis and vitiligo. J Am Acad Dermatol 2004;51:149-50.
[Google Scholar]
|
| 56. |
de Arruda LH, De Moraes AP. The impact of psoriasis on quality of life. Br J Dermatol 2001;144:33-6.
[Google Scholar]
|
| 57. |
Ashcroft DM, Li Wan Po A, Williams HC, Griffiths CE. Quality of life measures in psoriasis: a critical appraisal of their quality. J Clin Pharm Ther 1998;23:391-8.
[Google Scholar]
|
| 58. |
Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol 2004;51:704-8.
[Google Scholar]
|
| 59. |
Rakhesh SV, D'Souza M, Sahai A. Quality of life in psoriasis: a study from south India. Indian J Dermatol Venereol Leprol 2008;74:600-6.
[Google Scholar]
|
| 60. |
Gaikwad R, Deshpande S, Raje S, Dhamdhere DV, Ghate MR. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol 2006;72:37-40.
[Google Scholar]
|
| 61. |
Matto SK, Handa S, Kaur I, Gupta N, Malhotra R. Psychiatric morbidity in vitiligo and psoriasis: A comparative study from India. J Dermatol 2001;28:424-32.
[Google Scholar]
|
Fulltext Views
34,818
PDF downloads
4,615





