Translate this page into:
Pulmonary tuberculosis in association with Erasmus syndrome
2 Department of Radiodiagnosis, District Hospital, Bilaspur, Himachal Pradesh, India
Correspondence Address:
Mudita Gupta
Department of Dermatology, IGMC, Shimla, Himachal Pradesh
India
| How to cite this article: Sharma RK, Gupta M, Sharma AK. Pulmonary tuberculosis in association with Erasmus syndrome. Indian J Dermatol Venereol Leprol 2020;86:292-295 |
Sir,
The Erasmus syndrome is characterized by the association of exposure to silica with or without silicosis and the subsequent development of systemic sclerosis. This syndrome is named after the doctor who first described 17 cases of systemic sclerosis among gold miners in South Africa in 1957.[1] Silica-associated systemic sclerosis (Erasmus syndrome) is clinically, serologically and immunologically indistinguishable from idiopathic systemic sclerosis.[2] The association between silica exposure and tuberculosis has been studied since the beginning of the twentieth century.[3] Since then, various studies highlighted the development of tuberculosis in workers exposed to silica. This case study shows the development of pulmonary tuberculosis in a patient of systemic sclerosis after 26 years of silica exposure (Erasmus syndrome). There is only one study which has reported coexisting Erasmus and pulmonary tuberculosis.[4]
A 46-year-old man presented with slowly progressive binding down of the skin over the upper limbs, face, neck and upper part of chest for last 1 year. There was a history of bluish discoloration of fingers and toes on exposure to cold and finger-tip ulceration for 1 year. The patient also had mild chest pain and shortness of breath on exertion for 1 month. He complained of fever, low grade, off and on for 2 weeks. He gave a history of undocumented weight loss. There was no history of cough, hemoptysis, dysphagia, photosensitivity and joint pain. Moreover, there was no history suggestive of exposure to any other environmental pollutants that can cause scleroderma. The patient was a smoker for the last 30 years (1 packet/day). He was a stone cutter by occupation for the last 26 years. There was no family history of similar complaints. On examination, there was binding down of the skin over the face, bilateral hands, forearms, upper chest and legs (modified Rodnan score - 19/51). There was salt and pepper pigmentation over the upper part of the chest [Figure - 1]a. The bilateral hands had sclerodactyly [Figure - 1]b with digital pitted scars [Figure - 1]c, ragged cuticles without apparent nail fold telangiectasia. On auscultation of the chest, bilateral coarse crepitations were present. Examination of other systems was noncontributory. Complete hemogram was normal except for anemia (Hb - 9.8 gm%), and erythrocyte sedimentation rate (ESR) - 49 mm in the first hour. Liver and renal functions were normal. Human immunodeficiency virus, hepatitis B virus and hepatitis C virus serology were negative. Antinuclear antibodies by indirect immunofluorescence on hep-2 cells were positive (1: 320) speckled pattern, Scl-70 was also positive. Chest x-ray posteroanterior view revealed reticulonodular opacities over bilateral upper lobes [Figure - 2]a. Pulmonary function tests revealed moderate restriction (forced vital capacity-68% of the predicted, forced expiratory volume in one second-65% of the predicted). Computed tomography chest revealed mediastinal and hilar lymph nodes with peripheral egg-shell calcification [Figure - 2]b with small nodules predominantly over posterior and upper lobe on the left side [Figure - 2]c suggestive of silicosis. There was a small cavity in the left upper lobe suggestive of infective pathology [Figure - 2]d. Sputum for acid-fast bacillus and gene expert was negative. Bronchoalveolar lavage was done and gene expert was positive for acid-fast bacillus. Mantoux was 12 mm at 48 hours. Echocardiography showed trivial tricuspid regurgitation. Barium swallow was normal except for slight mucosal irregularity at the gastro-oesophageal junction. Skin biopsy on H and E staining [Figure - 3] showed sparse superficial and deep perivascular lymphohistiocytic infiltrate with marked thickening of collagen bundles in the reticular and papillary dermis. The thickened bundles were closely packed to give the hyalinised appearance, suggestive of scleroderma. On the basis of occupational history, clinical examination, serology, radiological investigations, gene expert from bronchoalveolar lavage and skin biopsy, the diagnosis of pulmonary tuberculosis in Erasmus syndrome (systemic sclerosis in association with silicosis) was made and patient was started on antitubercular therapy along with calcium channel blocker and advised to strictly stop smoking and avoid further exposure to silica and cold. Cyclophosphamide pulse was planned after 1 month.
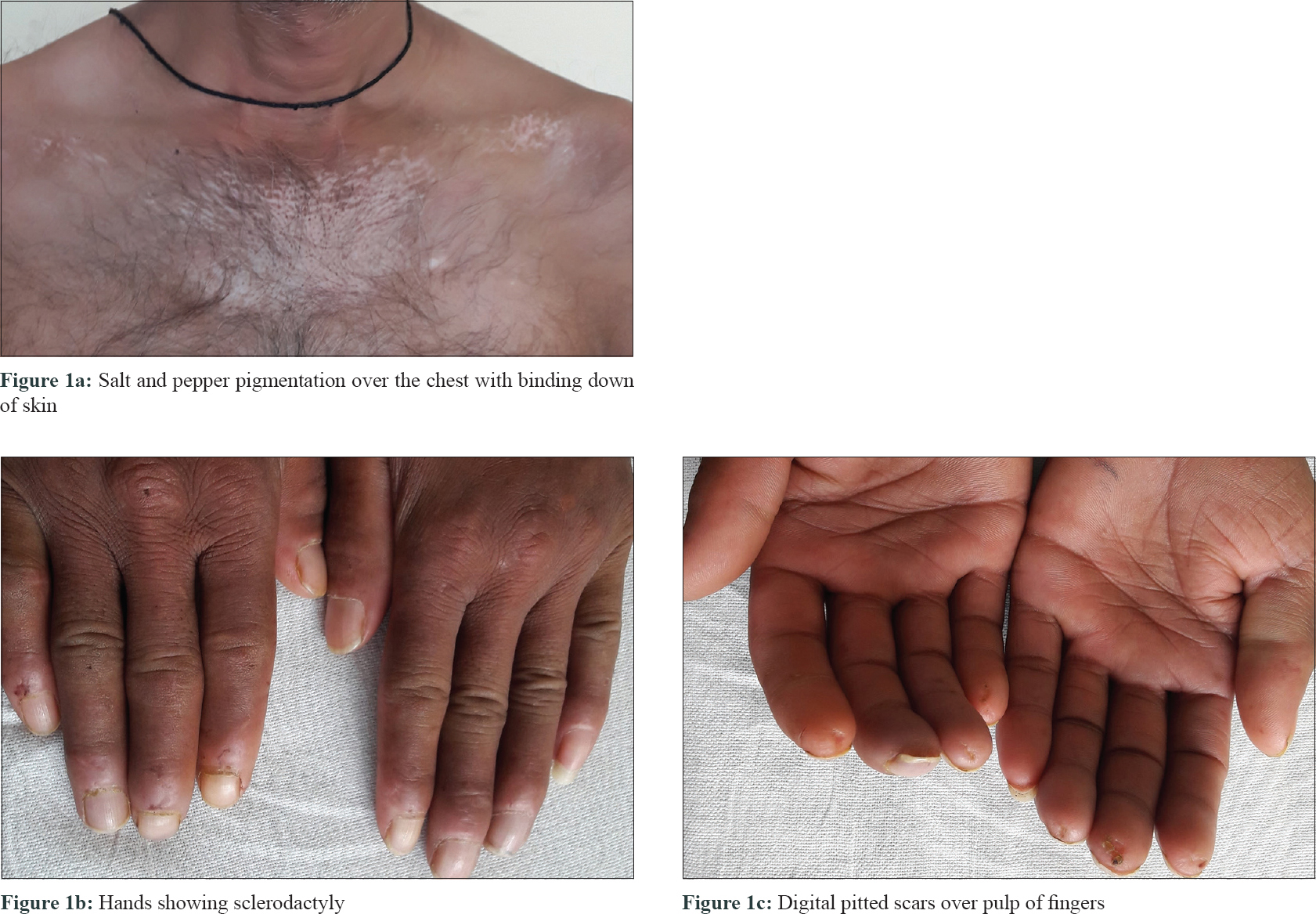 |
| Figure 1: |
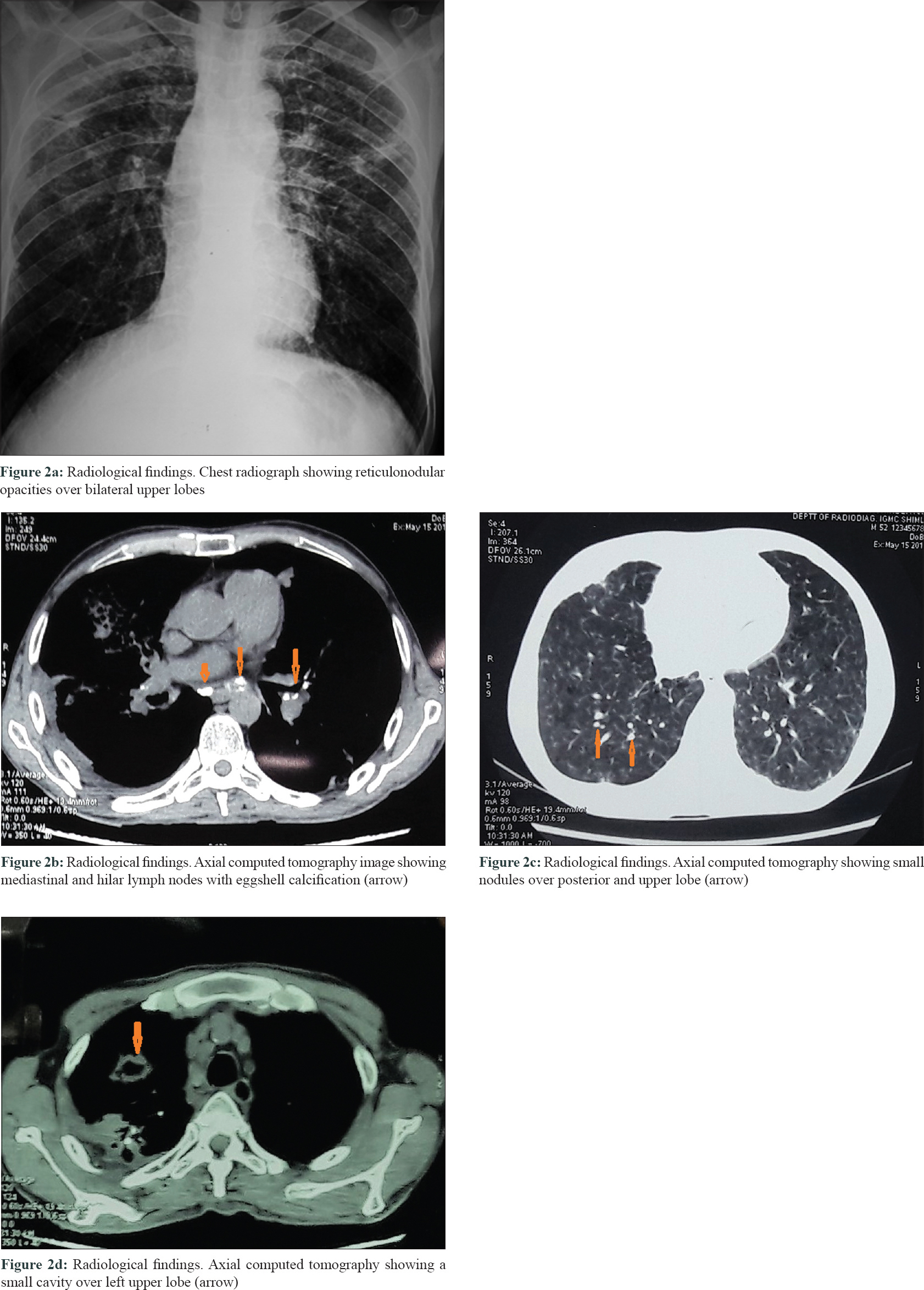 |
| Figure 2: |
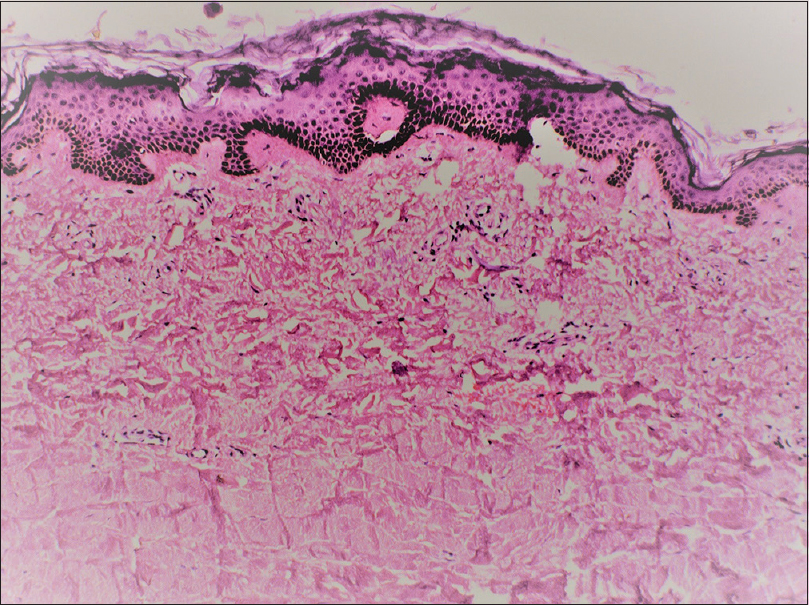 |
| Figure 3: Histopathology (H and E, ×400) showing hyalinization of dermal collagen |
Silicosis is a fibrosing lung disease caused by inhalation and deposition of crystalline silica particles, resulting in a pulmonary response. Silicosis or even exposure to silica without established disease is associated with increased risk of developing various pulmonary and systemic comorbidities including chronic obstructive pulmonary disease, tuberculosis, nontuberculous mycobacteria-related diseases, lung cancer, glomerulonephritis, rheumatoid arthritis, scleroderma and other autoimmune diseases.[3] The risk of a patient with silicosis developing tuberculosis increases with the duration and severity of exposure (2.8 to 39 times higher, depending on the severity of the silicosis than that found for healthy controls).[3]
Silica modifies the immune response of the lungs, impairs the function of pulmonary macrophages and with frequent exposure, causes macrophage apoptosis.[5] This leads to release of various cytokines (NF-kB and activator protein-1 are released triggering the production and release of inflammatory cytokines (TNF-, IL-1 and IL-6), proteases and arachidonic acid metabolites); hence fibrosis and local damage occurs and this predisposes to tuberculosis.[6] Another factor involved is surfactant protein A which appears at high levels in the bronchoalveolar lavage fluid of patients with silicosis. An excess of this protein seems to be associated with higher susceptibility to tuberculosis, possibly because it allows mycobacteria to enter the alveolar macrophages without triggering cytotoxicity and inhibits the formation of reactive nitrogen species by the activated macrophages.[3] It is also believed that the bacilli can remain encapsulated within the silicosis nodules which would be responsible for the reactivation of tuberculosis in such patients.[5] Smoking further aggravates the risk by increasing the level the surfactant protein-A.[7]
The silica dust which workers accumulate in their lungs during exposure is a lifelong risk for the development of pulmonary tuberculosis, even if silicosis is not present in the lungs. Furthermore, even after the exposure to dust ends, ex-workers continue to be at risk of developing silicosis and the development of silicosis places them at even greater risk of developing pulmonary tuberculosis.[8]
Due to the gravity of the problem, various disease-specific preventive and control measures were implemented in the United States which lead to a significant decline in silico sis-respiratory tuberculosis mortality during 1968–2006.[8]
There is controversial evidence of increased risk of pulmonary tuberculosis in idiopathic systemic sclerosis. Some studies report that there is an association between two diseases while others disagree with any such association.[9],[10]
Association of silicosis and systemic sclerosis is well known, due to diffuse pulmonary fibrosis. However, the development of tuberculosis is not an unexpected coincidence considering the high prevalence of tuberculosis in our country and the already damaged lung tissue. Surprisingly, there are not many existing reports of such cases which implies that these cases are under-recognised and underreported. This case highlights that the clinician should investigate the patient aggressively for tuberculosis to rule out such a possibility.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal the identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Bello S, Rinaldi A, Trabucco S, Serafino L, Bonali C, Lapadula G. Erasmus syndrome in a marble worker. Reumatismo 2015;67:116-22.
[Google Scholar]
|
| 2. |
Rustin MH, Bull HA, Ziegler V, Mehlhorn J, Haustein UF, Maddison PJ, et al. Silica-associated systemic sclerosis is clinically, serologically and immunologically indistinguishable from idiopathic systemic sclerosis. Br J Dermatol 1990;123:725-34.
[Google Scholar]
|
| 3. |
Barboza CE, Winter DH, Seiscento M, Santos Ude P, Terra Filho M. Tuberculosis and silicosis: Epidemiology, diagnosis and chemoprophylaxis. J Bras Pneumol 2008;34:959-66.
[Google Scholar]
|
| 4. |
Goyal A, Madan K, Singh N. Erasmus syndrome with pulmonary tuberculosis. BMJ Case Rep 2013;2013. pii: Bcr2013010443.
[Google Scholar]
|
| 5. |
Hnizdo E, Murray J. Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South African gold miners. Occup Environ Med 1998;55:496-502.
[Google Scholar]
|
| 6. |
Greenberg MI, Waksman J, Curtis J. Silicosis: A review. Dis Mon 2007;53:394-416.
[Google Scholar]
|
| 7. |
Nomori H, Horio H, Fuyuno G, Kobayashi R, Morinaga S, Suemasu K. Serum surfactant protein A levels in healthy individuals are increased in smokers. Lung 1998;176:355-61.
[Google Scholar]
|
| 8. |
Nasrullah M, Mazurek JM, Wood JM, Bang KM, Kreiss K. Silicosis mortality with respiratory tuberculosis in the United States, 1968-2006. Am J Epidemiol 2011;174:839-48.
[Google Scholar]
|
| 9. |
Ou SM, Fan WC, Chou KT, Yeh CM, Su VY, Hung MH, et al. Systemic sclerosis and the risk of tuberculosis. J Rheumatol 2014;41:1662-9.
[Google Scholar]
|
| 10. |
Pope JE, Goodwin JL, Ouimet JM, Krizova A, Laskin M. Infections are not increased in scleroderma compared to non-inflammatory musculoskeletal disorders prior to disease onset. Open Rheumatol J 2007;1:12-7.
[Google Scholar]
|
Fulltext Views
4,195
PDF downloads
2,565





