Translate this page into:
Pustular psoriasis: A distinct aetiopathogenic and clinical entity
Corresponding author: Dr. Biju Vasudevan, Department of Dermatology, Armed Forces Medical College, Wanowarie, Pune, India. bijuvasudevan1975@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vasudevan B, Das P, Bhatt S. Pustular psoriasis: A distinct aetiopathogenic and clinical entity. Indian J Dermatol Venereol Leprol. 2024;90:19-29. doi: 10.25259/IJDVL_542_2022
Abstract
Pustular psoriasis is a distinct subset of psoriasis that presents with involvement of the skin in the form of sterile pustules along with systemic manifestations. Though it has been conventionally grouped under the umbrella of psoriasis, recent research has shed light on its pathogenetic mechanisms associated with the IL-36 pathway, which is distinct from conventional psoriasis. Pustular psoriasis in itself is a heterogeneous entity consisting of various subtypes, including generalised, localised, acute, and chronic forms. There is confusion regarding its current classification as entities like deficiency of IL-36 antagonist (DITRA) which are closely related to pustular psoriasis both in their pathogenetic mechanism and its clinical manifestations, are not included under pustular psoriasis. Entities like palmoplantar pustulosis, which presents with similar clinical features but is pathogenetically distinct from other forms of pustular psoriasis, are included under this condition. Management of pustular psoriasis depends upon its severity; while some of the localised variants can be managed with topical therapy alone, the generalised variants like Von Zumbusch disease and impetigo herpetiformis may need intensive care unit admission and tailor-made treatment protocols. The advent of newer biologics and better insight into the pathogenesis of pustular psoriasis has opened the way for newer therapies, including tumour necrosis factor-alpha inhibitors, interleukin-1 inhibitors, interleukin-17 inhibitors, and granulocyte monocyte apheresis. It continues to be an enigma whether pustular psoriasis is actually a variant of psoriasis or an entirely different disease entity, though we feel that it is an entirely different disease process.
Keywords
Generalized pustular psoriasis
impetigo herpetiformis
infantile pustular psoriasis
pustular psoriasis in childhood
acrodermatitis continua of Hallopeau
Introduction
This article is a concise review of pustular psoriasis. An extensive review of the PubMed database was done with the MEDLINE terms “pustular psoriasis,” “generalized pustular psoriasis,” “pustular psoriasis in pregnancy,” “impetigo herpetiformis,” “pustular psoriasis in childhood,” “infantile pustular psoriasis,” “acrodermatitis continua of Hallopeau,” and “palmoplantar pustular psoriasis” until June 15, 2022, and relevant articles were included as part of this review.
Psoriasis is a chronic inflammatory dermatosis affecting the skin and various organ systems. It is heterogeneous with respect to natural history, age of onset, duration, morphology, host-environment interaction, triggers, and etiological factors. Out of the various morphological forms of psoriasis, pustular psoriasis is an uncommon entity with different pathogenesis, disease characteristics, and management implications. The generalised types of pustular psoriasis consist of acute generalized pustular psoriasis (von Zumbusch), sub-acute annular and circinate pustular psoriasis, acute generalized pustular psoriasis of pregnancy (impetigo herpetiformis), infantile pustular psoriasis, and juvenile pustular psoriasis.1 The localised types are Acrodermatitis Continua of Hallopeau (ACH) and Palmo-Plantar Pustulosis (PPP). The classification of pustular psoriasis is as provided in Table 1.1
| Generalized pustular psoriasis |
| Based on morphology and natural history |
| 1. Acute generalized pustular psoriasis |
| 2. Subacute annular and circinate pustular psoriasis |
| Based on age and precipitants |
| 1. Acute generalized pustular psoriasis of pregnancy (Impetigo herpetiformis) |
| 2. Infantile and juvenile generalized pustular psoriasis |
| Localized pustular psoriasis |
| 1. Palmoplantar pustulosis |
| 2. Acrodermatitis continua of Hallopeau |
Epidemiology
Pustular psoriasis is a rare disease, due to which there is a paucity of studies and minimal incidence data available in the literature. Its prevalence in French population in one study was estimated to be 0.64–1.76 per million.2 The median age of onset of generalized pustular psoriasis (GPP) is in the fifth decade, while infantile and juvenile pustular psoriasis occur in their respective age groups.3 Women are twice as predisposed as men for GPP.3 Acrodermatitis continua of Hallopeau (ACH) most commonly affects middle-aged females, though due to its rarity, limited studies are available to support this fact.4 The incidence of palmoplantar pustulosis is estimated to be 0.01–0.05%. Women are affected about five times more frequently as compared to men in this variant and incidence peaks between the age of 30 and 50 years.5
Etiopathogenesis
Studies suggest a different pathogenesis for de-novo pustular psoriasis versus pustular psoriasis occurring over a pre-existing chronic plaque-type psoriasis in GPP.6 A detailed description of the pathogenesis is shown in Figure 1. Mutations in IL-36RN have been found to have a central role in the pathogenesis of pustular psoriasis, and both familial and sporadic loss-of-function mutations of IL-36RN gene have been reported. The onset of GPP is significantly delayed in individuals with monoallelic mutations. This late onset of the disease is probably due to these individuals needing “multiple hits” in the form of known triggers. More than 50% of de-novo GPP harbor homozygous or compound heterozygous mutations in IL-36RN; however, recessive mutations in the same gene can cause GPP over pre-existing psoriasis vulgaris.7 A total of 25 pathogenic variants of IL-36RN mutations have been reported thus far. Out of them, c.115+6T>C is the most common in Asian populations.8 It is interesting to note that many healthy individuals may also carry homozygous mutations of c.115+6T>C in the IL-36RN gene, or such subjects may develop GPP only later in adulthood. Thus, the genetic basis for GPP is thought to be oligogenic rather than monogenic. In a study done on two groups of patients, one who had only GPP and the other who had GPP along with psoriasis vulgaris (PV), all 11 patients with GPP only had one of the pathogenic variants of IL-36RN, whereas only 2 out of the 20 patients of GPP and PV had a similar mutation.9 Similarly, a meta-analysis of 233 patients with GPP showed IL-36RN mutations were associated with earlier onset of disease and a lower prevalence of psoriasis vulgaris.10 Another interesting fact to note is that the IL-36RN mutation is more consistently associated with fever and systemic symptoms, and mutations in the same gene, more specifically the c.115+6T>C variant, is associated with a geographical tongue.11,12

- Keratinocytes express pro-inflammatory cytokines IL-36α, IL-36β, and IL-36γ, which bind to their respective receptors on keratinocytes, dendritic cells, resident T-cells, and monocytes stimulating an inflammatory response by activation of neutrophils, macrophages, T-cells, and dendritic cells through a complex cascade. IL-36Ra (IL-36 Receptor antagonist) is the key cytokine that counter-balances this cascade and is encoded by the gene IL-36RN. Loss of function mutation of IL-36RN causes functional impairment of IL-36Ra, in turn leading to uncontrolled disinhibition of the downstream inflammatory cascade like N F-ҡB and mitogen-activated protein kinase signaling pathways leading to unopposed inflammation in the skin manifesting with various clinical features of GPP.
On the other hand, CARD14 mutations predispose individuals with psoriasis vulgaris to develop pustular psoriasis, and is rarely seen with GPP alone.13 A total of 10 genetic mutations have been described that are associated with PV. Not all mutations are common in patients with GPP. Only, c.526G>C missense mutation has been found in GPP patients and is also seen in individuals with Asian ancestry.14 Some gene expression studies have suggested that the proteome of GPP is similar to that of plaque psoriasis, but is skewed toward the innate immune pathway, whereas those with plaque psoriasis show immune responses leaning towards adaptive immunity.
Another gene, AP1S3, which codes for the σ1C subunit of the adapter protein (AP-1) stabilizes the AP-1 hetero-tetramer and is involved in vesicular trafficking between the Golgi complex and the endosomes. Any loss of function of such a gene leads to the loss of keratinocyte autophagy and abnormal accumulation of p62, which leads to NF-kB activation and upregulation of inflammatory cytokines.15,16
The mechanisms associated with CARD14, IL-36RN, and AP1S3 in GPP have been elucidated in Figure 2.
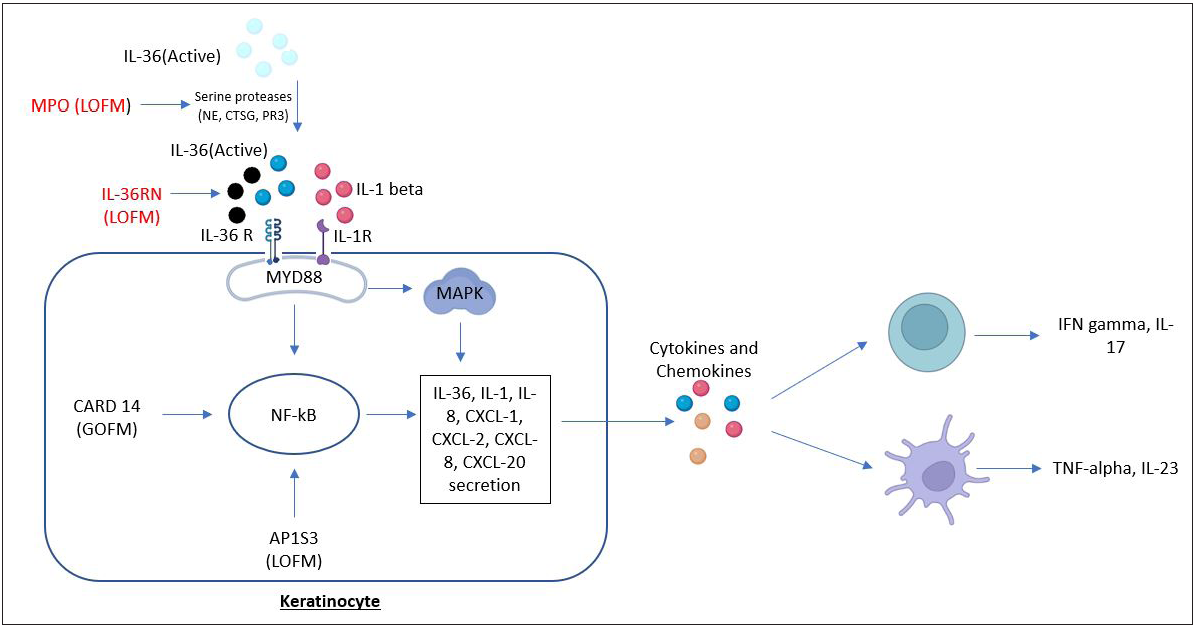
- Pathways and processes induced by IL-36RN, CARD14, and AP1S3. Loss-of-function mutations in both IL-36RN and MPO genes cause upregulation of IL-36 signaling. IL-36RN mutation cause inability of IL-36Ra to antagonize and limit the pro-inflammatory effects of IL-36, and the MPO gene mutation led to upregulate the activity of NE, CTSG, and PR3. These three are serine proteases that cleave IL-36 precursors into pro-inflammatory forms. Increased IL-36 signaling activates the downstream pro-inflammatory NF-κB and MAPK pathways by binding to IL-36 receptor, leading to the secretion of chemokines/cytokines (IL-36, IL-1, IL-8, CXCL1, CXCL2, CXCL8, CXCL20) from the keratinocyte and resulting in the activation of neutrophils, T cells, and dendritic cells. Gain-of-function mutations of CARD14 and AP1S3 loss-of-function mutations hyperactivate NF-κB pathway and are also involved in the processes of inflammatory responses. MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor-κB; MAPK, mitogen-activated protein kinase; LOFM, loss-of-function mutations; GOFM, gain-of-function mutations
While plaque psoriasis is driven by TNF-α/IL-17/IL-22/IL-23 inflammatory pathways; loss-of-function mutation of IL-36Ra leading to unopposed activation of IL-36R is now proven to be the key reason behind the pathogenesis of pustular psoriasis. Gene expression profiling of skin biopsies from plaque psoriasis lesions by microarray analysis found increased expression of TNF-α, IL-17A, IL-1, IL-36, and IFNs; whereas pustular psoriasis lesions showed higher expression of IL-36α, IL-36γ, and IL-1β and lower expression of IFNγ and IL-17A than plaque psoriasis.17 Pustular psoriasis also showed increased expression of chemokines directed at neutrophils, for example, CXCL1, CXCL2, and CXCL8, as compared to plaque psoriasis lesions.6
Skin derived anti-leucoproteinase (SKALP), also known as elafin, is an inducible inhibitor of neutrophil derived elastase. Measurement of its level from epidermal scales and biopsies shows it to be markedly decreased as compared to normal individuals and patients with plaque psoriasis. This can also be one of the reasons for the formation of epidermal pustules seen in pustular psoriasis.
Environmental triggers like withdrawal of systemic corticosteroids and sometimes cyclosporine, upper respiratory tract infections, pregnancy, hypocalcemia, psychological stress, use of topical agents such as coal tar, dithranol, and corticosteroids under occlusion are known to precipitate pustular psoriasis. Also, drugs like NSAIDs, beta-blockers, lithium, terbinafine, potassium iodide, phenylbutazone, and bupropion are implicated as triggers.18 TNF-α inhibitors, for example, infliximab, adalimumab, etanercept, certolizumab pegol, and golimumab, are known to trigger paradoxical pustular psoriasis.19
Impetigo herpetiformis is considered to occur as a result of physiological and metabolic changes due to pregnancy, along with other underlying etiological factors like hypocalcemia, hypoparathyroidism, bacterial infections, and medications.20
Etiology of PPP includes hypocalcemia, hypoparathyroidism, bacterial infections, stress, and certain medications.20 Here, the acrosyringium, which is the intraepidermal part of the eccrine sweat duct, acts as a primary site of inflammation, which is mediated by Langerhans cells and IL-17.21 This is evidenced by the immunostaining for gross cystic disease fluid protein 15 (GCDFP-15) and epithelial membrane antigen (EMA) which are eccrine markers seen localised to the cells lining the intraepidermal vesicles.22 The role of nicotine in the causation of PPP appears to be due to its ability to induce neutrophils to release IL-8.21 Also in smokers, there is increased expression of the nicotinic acetyl choline receptor (nAchR).23
Like in GPP, IL-36RN, and CARD14 mutations are implicated in ACH too.4 ACH is typically triggered by local trauma or infection; however, inflammatory and neural etiologies have also been suggested.24
Clinical Features
Generalized pustular psoriasis
Acute GPP (Von Zumbusch) is characterised by the sudden onset of burning sensation and erythema of the skin, followed by pustulation occurring either de novo or over existing psoriatic lesions. Sheets of pustulation and erythema spread progressively, recruiting previously unaffected skin [Figure 3]. It can sometimes lead to erythroderma. The pustulation appears in crops, quickly coalescing to form lakes of pus that later exfoliates, leading to the simultaneous presence of lesions in different stages of evolution. The configuration of the pustules may vary from isolated ones to circinate, collarette, and even lakes of pus. The tongue may particularly be involved, showing a geographic pattern. Apart from the regular nail findings in psoriasis vulgaris like thimble pitting, sub-ungual hyperkeratosis, curvature abnormalities, discoloration, and thickening of the nail plate; in pustular psoriasis, the sub-ungual pustules can cause onycholysis, onychomadesis, and complete destruction of the nail apparatus.25

- Generalized pustular psoriasis (Von Zumbusch) with extensive pustulation developing denovo.
GPP is associated with systemic manifestations like high-grade fever, severe malaise, and arthralgia.26 Increased fluid loss and dehydration may precipitate acute kidney injury as well as cardiovascular shock. Sudden loss of plasma proteins into the tissues and malabsorption can cause hypoalbuminemia which in turn may precipitate hypocalcemia. Aseptic interstitial pneumonitis presenting with acute onset breathlessness and hypoxia and consequent acute respiratory distress syndrome due to pustular psoriasis is rare, but life-threatening.27 Other manifestations include arthritis, uveitis, otitis media, and epigastric pain.27 Post-inflammatory hyperpigmentation, telogen effluvium, and amyloidosis may occur as late sequelae.28
The mortality rate is below 5% in GPP; mostly due to multi-organ failure and disseminated intravascular coagulation.27 Multiple studies suggest that patients with extensive mucosal involvement and co-morbidities have a higher risk of mortality.28
Laboratory abnormalities like neutrophilic leukocytosis with a shift to the left, elevated C-reactive protein (CRP), hypoalbuminemia, and hypocalcemia can be seen.29 Recent studies have shown that a higher percentage of GPP patients have abnormal liver function tests.30 Magnetic resonance imaging (MRI) has shown corroborating evidence of cholangitis with alternate dilatations and strictures of the biliary tracts, with histopathology showing dense neutrophilic infiltration.30 This characteristic liver and biliary tract involvement is called neutrophilic cholangitis (NC).
Subacute annular and circinate generalized pustular psoriasis is characterised by lesions that start with discrete areas of erythema, later becoming edematous and raised with slow central clearing. Pustules appear on the advancing edge of the lesion, which leaves behind a trail of scales on drying (Figures 4–5). There are usually no systemic symptoms. Similar lesions as seen in generalized pustular psoriasis may occur. It may also be a common presentation of pustular psoriasis during infancy and early childhood. Recurrent circinate erythematous psoriasis is a variant wherein there is no pustulation, but histopathologically spongiform pustules are noticed.

- Large annular plaque developing pustulation in subacute annular and circinate generalized pustular psoriasis.

- Close up view of subacute annular and circinate generalized pustular psoriasis.
Infantile and juvenile generalized pustular psoriasis is a very rare entity and accounts for only for about 1% of cases of severe psoriasis in childhood. It is twice as common in males as compared to females. It can occur at any age but is more common below one year of age. Triggering factors include infection, vaccinations, and steroids. About 25% of patients have a positive family history of psoriasis, and psoriasis occurs subsequently in about 59% of individuals. In this age group, systemic symptoms are characteristically absent. The lesions are initially localised over the folds and flexures before widespread dissemination occurs. Beylot et al., in a case report of 27 children with generalized pustular psoriasis, classified it into three groups: von Zumbusch, annular, and mixed. Annular and circinate lesions occur more frequently in juvenile cases as compared to adults.31
Pustular psoriasis of pregnancy was first described by Von Hebra in 1872 and is popularly known as “Impetigo Herpetiformis.” It is most commonly seen in the third trimester, though cases occurring as early as in the first month of gestation have been reported.20 It remits rapidly in the postpartum period. It has a tendency to relapse with subsequent pregnancies, with the disease occurring earlier and being associated with more morbidity in subsequent pregnancies. It may occur de novo or with an antecedent history of psoriasis. The lesions start in the inguino-genital folds and then can progress to involve the whole body, showing a picture similar to GPP [Figure 6]. Constitutional symptoms, including fever, arthralgia, tachycardia, diarrhea, and seizures can be present. Common laboratory anomalies include neutrophilia, increased erythrocyte sedimentation rate, hypocalcemia, hypophosphatemia, hypoalbuminemia, and decreased vitamin D levels.32 Hypocalcemia is attributed to hypoalbuminemia due to the decrease in the half-life of albumin from 11 days to 4 days as a result of the loss of plasma proteins.33 Decreased vitamin D has also been noted in patients, further complicating hypocalcemia. Bajaj et al. have reported success in the treatment of impetigo herpetiformis with supplementation of vitamin D and calcium.34 Since the above abnormalities are found in only a few patients, generalization of the above findings should be avoided.

- Pustules and crusted lesions on abdomen and thighs in impetigo herpetiformis.

- Acrodermatitis continua of hallopeau with pustules on nail folds and nail changes.
Controversy exists regarding whether impetigo herpetiformis is a distinct entity or a variant of pustular psoriasis. Pustular psoriasis occurring in pregnancy can be classified into two distinct subsets. First, generalized pustular psoriasis of pregnancy, which is mostly seen in patients who already have a history of pustular psoriasis or psoriasis vulgaris. Here the lesions are more generalised and associated with systemic abnormalities, as seen in generalized pustular psoriasis. The second variant, which is the true impetigo herpetiformis, occurs for the first-time during pregnancy, and recurs with each successive pregnancy. Patient is free from skin disorder in pregnancy. Lesions here are mostly annular or polycyclic plaques with pustules in the periphery of the lesion. Also, response to steroids varies between the subsets, with generalized pustular psoriasis of pregnancy showing an excellent response to steroids whereas impetigo herpetiformis showing a low to moderate response.35 Histopathological differences have also been noted between the variants, with generalized pustular psoriasis of pregnancy showing dilatation of the papillary blood vessels and multiloculated spongiform pustules containing predominant neutrophils, whereas true impetigo herpetiformis shows no such dilation of the blood vessels, spongiform pustules showing no confluence, and the pustules showing both neutrophils and mononuclear cells.36
Localized Pustular Psoriasis
Acrodermatitis continua of Hallopeau (ACH) may follow local trauma or infection; however, such a history may not be present at all times.4 The first symptom of ACH is usually painful erythema starting from the tip of the digit, followed by the development of painful pustules around and below the nail bed & matrix, causing onychodystrophy and anonychia [Figure 7]. Over time, ACH shows proximal progression, gradually involving parts of the hand or foot. Late complications include osteitis and acro-osteolysis, which cause an immense negative impact on the quality of life.37 Confusion exists with terminologies describing pustular eruptions of the tips of fingers and toes. Acrodermatitis continua of Hallopeau is a more aggressive disease with intermittent eruptions without periods of complete remission, a more generalised disease, and progressive worsening of the disease characterised by acro-osteolysis. A more benign form was described by Radcliffe and Crocker, and has also been termed dermatitis repens or acropustulosis repens. It is a more localised form characterised by one or a few pustules on fingers and toes, with asymptomatic phases between the flares, and without any permanent structural damage.38 Another term, parakeratosis pustulosa, defines a condition occurring exclusively in females below five years of age with transient acral pustules followed by erythemato-squamous lesions of the periungual skin with pitting and ridging of the affected nail plate.39

- Palmoplantar pustulosis (Pustules on the sole of right foot).
Palmoplantar pustulosis is a controversial entity whose association with psoriasis is debated. Though it is found to be associated with psoriasis more than just by chance, it can occur in isolation too [Figure 8]. Cigarette smoking is strongly associated with palmoplantar pustulosis with up to 95% of patients with PPP having a positive history of smoking.21 Apart from this, many other inciting factors like stress (43% patients),40 nickel sensitivity (3% patients),41 infections like tonsillitis, dental infections, the Koebner phenomenon, and hot and humid conditions have been mentioned.32 A paradoxical flare of palmoplantar pustulosis with the anti TNF-alpha agents is also noted.19 Pustular eruptions of the hands and feet have been described in literature with many overlapping terminologies. Palmoplantar pustular psoriasis (PPP) or the Ingram – barber type PPP is associated with family history of psoriasis, association with HLA B13 and B17 and a better response to methotrexate, whereas the other form, termed pustulosis palmaris et plantaris shows no such association but instead is associated with skeletal alterations like chronic multifocal osteomyelitis, especially of the sternoclavicular bones.
Infantile acropustulosis is characterised by recurrent crops of pruriginous pustules occurring in infants, most commonly in response to a hypersensitivity reaction to scabies. It is generally self-limiting and regresses completely by 6 months.38
Differential Diagnosis
Acute generaliszd exanthematous pustulosis (AGEP) is perhaps the closest differential diagnosis of acute GPP, triggered by drugs, particularly pristinamycin (an anti-staphylococcal drug used in Europe) and aminopenicillins.42 Other drugs implicated are fluoroquinolones, sulfonamides, hydroxychloroquine, ketoconazole, terbinafine, and fluconazole. AGEP is differentiated by usually having a prior history of drug intake, predominant flexural involvement, a short duration of symptoms, an absence of a previous history of psoriasis, and an absence of joint pain.43 Other differential diagnoses are extensive impetigo, staphyloderma, extensive candidiasis in the immunosuppressed, Sweet’s syndrome, the pustular variant of pyoderma gangrenosum, the Bowel Associated Dermatitis Arthritis Syndrome (BADAS syndrome), Behcet’s syndrome, and subcorneal pustular dermatosis.3
Differentials of ACH include bacterial, fungal, or viral paronychia; onychomycosis, dyshidrotic eczema, or contact dermatitis that has been secondarily infected. Further, peri-ungual scaling, erythema of connective tissue disorders, and malignancies like Bowen’s disease, squamous cell carcinoma, and melanoma need to be ruled out.
Diagnosis and Severity Classification
Based on history, clinical examination, and histopathological correlation; Umezawa et al. had proposed a diagnostic criterion for generalized pustular psoriasis.44 All five criteria need to be present for diagnosis and include: (a) constitutional symptoms–fever, generalized malaise, (b) aseptic pustules in generalized distribution, (c) characteristic histopathology with Kogoj spongiform pustules, (d) laboratory confirmation by leukocytosis with shift to left, raised ESR, positive CRP, high ASO titres, or hypocalcemia, and (e) recurrences. The medical board of the National Psoriasis Foundation has adopted a severity classification by Ohkawara et al. to determine the management approach.45 First, the skin symptoms are calculated as follows:
Erythema—1 point for each part of the body involved, 2 points for 50% body involvement, and 3 points for generalised involvement
Pustules—1 point for each part of the body involved, 2 points for 50% body involvement, and 3 points for generalised involvement
1 additional point each for confluent pustules and the presence of enanthem
0–2: mild
3–6: moderate
7–10: severe.
Histopathology
In GPP, the early stages of the disease show a lymphocytic predominant infiltrate, which is later replaced by neutrophils. Intense infiltration causes papillary edema and spongiosis. Deeper down in the dermis, there are dilated blood vessels with fewer neutrophils. Masses of neutrophils coalesce to form spongiform pustules of Kogoj, which swiftly become macroscopic. When epidermal turnover gets accentuated, the sub-corneal pustules are shed, which histo-pathologically correlate with parakeratosis. Acanthosis and elongation of rete ridges are common in psoriasis vulgaris. AGEP also shows histopathological similarity with GPP in the form of neutrophilic pustules, spongiosis, and papillary dermal edema, though rarely dermal eosinophils can be seen, which are not seen in GPP. Single cell necrosis and features of vasculitis may be rarely seen in AGEP.
In ACH, apart from the expected changes as seen in GPP, the nail matrix is mostly involved, which shows moderate acanthosis, spongiosis, and lymphocytic as well as neutrophilic exocytosis.47
Treatment
Acute GPP should be managed in an institutional setting, preferably in a dermatology ICU. Infections, if present, are treated with suitable antibiotics/antivirals and any identifiable triggers like systemic drugs and topical irritants should be withdrawn. Abrupt discontinuation of drugs like cyclosporine or systemic/topical steroids should be avoided. Other triggers, like psychological stress and hypocalcemia, should be corrected. The patient should be nursed at an ambient temperature to prevent excessive loss of heat, fluid, and electrolytes. Adequate hydration is to be maintained by ensuring the requisite oral intake dictated by 24-hour urine output. Associated electrolyte imbalances should be actively looked for and corrected promptly. For correction and prevention of hypoproteinemia, a protein diet of 1–1.5 g/kg/day is administered. Topical therapy in the form of bland emollients is used. Skin hygiene and hydration can further improved by soothing normal saline compresses and soaks.
A summary of the systemic therapy is given in Table 2. Acitretin has been recommended as the first-line therapy in adults for GPP. Being teratogenic, it cannot be used during pregnancy or by individuals planning a family. Patients usually respond within a week to a fortnight of starting oral retinoids. Despite its ineffectiveness, isotretinoin has been anecdotally used successfully because of its significantly shorter half-life as compared to acitretin.48 Cyclosporine (CysA) has a “rapid cool down” effect on pustular psoriasis. Another regimen is sequential therapy with CysA and acitretin which consists of three phases: the clearing phase where CysA is started at the maximum therapeutic dose; the transition phase, where acitretin is introduced and CysA tapered off and the continuation phase in which the patient is maintained on acitretin alone or with UVB(Re-UVB) or PUVA(Re-PUVA). Methotrexate (Mtx) is used in individuals with GPP in whom systemic retinoids are contraindicated or are unresponsive to acitretin. Depending on disease severity, the effective dose of methotrexate may range from 5 to 15 mg/week. Although weekly methotrexate doses up to 50 mg/week have been used in the past, recent recommendations cap weekly dosing to no more than 25 mg. However, in cases of severe GPP, Mtx use is limited by a relatively slower onset of action, reduced efficacy (60%), erratic absorption, and clearance.49 In patients with sub-optimal response to oral methotrexate, weekly subcutaneous injections may be tried for better bioavailability. Systemic steroids are to be avoided and only given to tide over life-threatening emergencies like acute respiratory distress syndrome and pustular psoriasis of pregnancy, in which they remain the treatment of choice.50
| S. No | Drug (Level of evidence) | Dosage | Route | Remarks |
|---|---|---|---|---|
| First line | ||||
| 1. | Acitretin (1B) | 0.75–1 mg/kg/day, Maintenance: 0.125–0.25 mg/kg/day | Oral | Rapid onset of action |
| 2. | Cyclosporine (4) | 3–5 mg/kg for 2–3 months, tapered 1 mg/kg every fortnightly | Oral | Rapid onset of action |
| 3. | Infliximab (4) | 5 mg/kg at 0, 2, 6 weeks followed by 8 weekly | IV | Rapid onset of action |
| 4. | Methotrexate (4) | 5–15 mg/week. Initially 7.5 mg /week, increase by 2.5 mg/week | Oral | Slower onset of action |
| Second line | ||||
| 5. | PUVA (4) |
Oral 8-MOP: 0.6–0.8 mg/kg. Topical: Methoxsalen lotion |
Oral/Topical | Topical: localised, Oral: generalised |
| 6. | Corticosteroids (4) | Prednisolone 0.5–1 mg/kg | Oral | Short duration – acute emergencies, pregnancy |
| Other biologics | ||||
| 7. | Adalimumab (4) | 40 mg/week × 2 weeks, then 2 weekly | SC | - |
| 8. | Etanercept (4) | 0.8 mg/week; 25 mg | SC | Pediatric use, juvenile pustular psoriasis |
| 9. | Certolizumab pegol (2) | 200 mg every 2 weeks | SC | Safe in pregnancy, no placental transport – lack of Fc receptor. |
| 10. | Anakinra (2) | 100 mg OD | SC | |
| 11. | Canakinumab (4) | 150 mg 8 weekly | SC | |
| 12. | Secukinumab (4) | 300 mg at 0, 1, 2, 3, 4 weeks, followed by monthly doses | SC | |
| 13. | Ixekizumab (4) | Two 80 mg initial doses then 80 mg 4 weekly | SC | |
| Topical | ||||
| 14. | Topical therapy (4) | Steroids, calcipotriene, tacrolimus – once to twice daily | Local | |
| Other therapies | ||||
| 15. | Apremilast (4) | 10 mg/day increased to 30 mg BD | Oral | |
| 16. | Granulocyte and monocyte adsorption apheresis (5) | 60 minutes sessions twice weekly, total of 7–8 sessions | ||
Second-line therapy comprises biologics like TNF-α inhibitors and IL-17 inhibitors. Among the biologics, owing to its rapid onset of action, infliximab is considered the first-line therapeutic option in patients with acute GPP. Etanercept and adalimumab have also been used. IL-17 inhibitors like secukinumab and ixekizumab have also gained wide acceptance, and their efficacy is backed by trials.51
PUVA (Psoralen plus UV-A) is successfully used in well-controlled GPP to maintain the remission. Since the onset of action of PUVA is slow, PUVA does not have a role in the acute stages of pustular psoriasis.
The role of the standalone PDE4 inhibitor apremilast in pustular psoriasis is yet to be backed by studies, but a case with multiple comorbidities has been managed successfully.52
Due to the recent discovery of the role of IL-1 in GPP pathogenesis, IL-1b inhibitors, like canakinumab and gevokizumab have been tried with satisfactory outcomes; but further RCTs are required to evaluate their efficacy and safety.53 IL-1Ra (IL-1 receptor antagonist) anakinra is another promising molecule which has shown good results in isolated case reports, and future placebo-controlled RCTs are in the pipeline.54
In recalcitrant cases of GPP as well as in impetigo herpetiformis, more recent intensive interventions like granulocyte and monocyte adsorption apheresis (GMA), which works by extracting activated WBCs, resulting in a drop in TNF and IL levels.55 A recent case report has shown improved outcomes for disease in the mother and the health of the fetus with the use of GMA.56
Approaches relating to direct inhibition of IL-36 pathway were tested in mouse models. An oral small molecule, A 552, has shown to attenuate IL-36γ induced CXCL 1 release in a dose-dependent manner.57 The EFFISAYIL 1 Phase 2 multicenter randomised double-blind placebo-controlled trial has tested Spesolimab, an anti-IL-36 R monoclonal antibody for GPP. In this trial, 53 patients with GPP were treated with a single dose of spesolimab or placebo for a 12-week period. 54% patients in the spesolimab group showed no visible pustules as compared to 6% of patients in the placebo and 43% of patients in the spesolimab group showed completely clear skin, compared to 11% in the placebo group. The rate of non-serious infections was higher with spesolimab as compared to placebo, and two patients developed a drug reaction with eosinophilia and systemic symptoms.58 Two other trials, namely the EFFISAYIL 2 trial and EFFISAYIL ON trials are underway. The former aims at investigating Spesolimab as a maintenance treatment to prevent the flares of GPP, and the latter is a 5-year-long trial to investigate the long-term efficacy and safety of the drug.
Another IL-36 R monoclonal antibody named Imsidolimab is currently being investigated in a phase 2 trial (GALLOP trial) for the patients of GPP. The trial has shown promising results with 6 out of the 8 patients having achieved the primary end point (response on the clinical global assessment scale) at the end of the 4 and 6 week study periods. Following this, a phase 3 GEMINI-1 clinical trial has been initiated.59
While mild cases of impetigo herpetiformis can be managed with topical steroids, vitamin-D analogues, and NBUVB phototherapy; immunomodulators may be required for severe cases. As systemic retinoids are contraindicated in pregnancy, conventional first-line therapy is limited to oral corticosteroids; however, cyclosporine and infliximab may also be used. Some of the known pregnancy and fetal complications of steroids are gestational diabetes, pre-eclampsia, macrosomia, low birth weight, oral clefts, premature rupture of membranes, and pre-term birth. Cyclosporine undergoes placental transfer and has been associated with pre-eclampsia, pre-term delivery, early rupture of membranes, and low birth weight. Nursing mothers on CysA should not breastfeed their babies. Although impetigo herpetiformis is known to remit in the post-partum period, methotrexate or retinoids can be used in the cases that persist post-delivery with the advice of avoiding breastfeeding.
First-line treatment for children with GPP is similar to that for adults, that is, cyclosporine, methotrexate, acitretin, and etanercept. Systemic retinoids may not be the ideal first-line treatment option in children, since they can cause premature epiphyseal closure. Cyclosporine is well tolerated by children, is known to cause fewer side effects, and is often the first treatment used before retinoids. Methotrexate is contraindicated in children under the age of 2 years. PUVA too should not be used in children under the age of 12 years.
ACH and PPP have a chronic and often recalcitrant course. Topical agents that have been tried with varying results include corticosteroids, calcineurin inhibitors, vitamin D analogs, 5-fluorouracil, and tar preparations. Systemic agents like methotrexate, retinoids, cyclosporine, phototherapy, or photochemotherapy have been used, with no particular modality being better than the other. In recent years, biologics like TNF-α inhibitors, IL-17 antagonists, and IL-12/23 inhibitors have been used successfully to provide long-term remissions.60 Targeted phototherapy like Excimer laser/light holds promise for treatment as well as remission in ACH and PPP.
There are currently very few Indian studies that shed light on the genetic profile or management strategies of patients with pustular psoriasis in India. A prospective study by Sudha Rani Chintagunta et al. studied 14 patients with generalized pustular psoriasis over a 2-year period. Out of them, 10 were of the acute type, 3 were pregnancy-related, and 1 childhood onset. Most of the patients were managed with oral steroids followed by maintenance with acitretin/methotrexate. One patient with tuberculosis was managed with cyclosporine. Another patient with HIV positive status was managed with Anti-Retroviral Therapy and Acitretin. In one patient, the lesions resolved with antibiotics only.61
Disease Burden
Pustular psoriasis, specifically the pustular variant, poses severe disease burden on the patient. The flares of GPP are more severe and potentially life threatening as compared to the exacerbations of PV and have a huge impact on the physical, mental health and quality of life (QoL) of the patients.62 According to a single center, retrospective analysis of records by Choon et al., mean DLQI scores of 102 patients with GPP over a 5-year period ranged from 12 to 17, suggesting a high impact of GPP on QoL.63 The same study also described the mean hospital stay of GPP patients to be 10.3 days. A case control study from Sweden comparing the economic burden of patients with GPP and patients with PV without GPP revealed that the economic burden of pustular psoriasis is about 1.8 times higher as compared to PV.64
Conclusion
Pustular psoriasis is a unique subset of psoriasis that differs not only in appearance but also in etiopathogenesis and treatment approach. With the new insights into the genetic and molecular basis of its occurrence, newer targeted monoclonal antibodies are in trial phase of treatment. More studies are required especially in Indian patients to learn about the mutations harbored by Indian patients with pustular psoriasis. With different mutations like IL-36 RN and CARD 14 having different connotations as far as progression, symptoms, and targeted therapies are concerned, widespread genetic testing for the patients with pustular psoriasis can be implemented. All in all, it indeed needs to be treated like a totally different entity as compared to psoriasis vulgaris. While conventional acute care with tried and tested therapies remains the cornerstone in the management of pustular psoriasis, the recent advances in molecular pathogenesis and evolving therapeutics are indeed exciting, promising a great future for revealing this rare entity much more in the coming days.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645-51.
- [CrossRef] [PubMed] [Google Scholar]
- Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-73.
- [PubMed] [Google Scholar]
- Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acrodermatitis continua of Hallopeau: clinical perspectives. Psoriasis Targets Ther. 2019;9:65-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pustulosis palmoplantaris is a disease distinct from psoriasis. J Dermatol Treat. 2011;22:102-5.
- [CrossRef] [PubMed] [Google Scholar]
- ”Autoinflammatory psoriasis”—genetics and biology of pustular psoriasis | Cellular & Molecular Immunology [Internet]. [cited 2020 Sep 10]. Available from: https://www.nature.com/articles/s41423-020-0519-3?elqTrackId7eca817118ff42bb93f2b893218725dd
- IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mutation analysis of the IL36RN gene in 14 Japanese patients with generalized pustular psoriasis. Hum Mutat.. 2013;34:176-83. doi: 10.1002/humu.22203
- [CrossRef] [PubMed] [Google Scholar]
- The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol.. 2013;133:2514-21.
- [CrossRef] [PubMed] [Google Scholar]
- IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol.. 2015;135:1067-1070.e9.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of IL36RN and CARD14 mutations with clinical manifestations and laboratory findings in patients with generalised pustular psoriasis. Indian Journal of Dermatology, Venereology and Leprology. 2022;17:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in IL36RN are associated with geographic tongue. Hum Genet. 2016;136:241-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Frontiers | Clinical and Genetic Heterogeneity of CARD14 Mutations in Psoriatic Skin Disease | Immunology [Internet]. [cited 2020 Sep 10]. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02239/full
- An update on genetic basis of generalized pustular psoriasis (Review) Int J Mol Med. 2021;47:118.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- AP1S3 mutations cause skisn autoinflammation by disrupting keratinocyte autophagy and up-regulating IL-36 production. J Invest Dermatol. 2016;136:2251-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Critical Role of the IL-1β–IL-1R Signaling Pathway in Skin Inflammation and Psoriasis Pathogenesis. J Invest Dermatol. 2019;139:146-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An update on generalized pustular psoriasis [Internet]. [cited 2020 Sep 11]. Available from:https://www.tandfonline.com/doi/full/10.1080/1744666X.2019.1648209
- Paradoxical Reactions: Anti-Tumor Necrosis Factor Alpha Agents, Ustekinumab, Secukinumab, Ixekizumab, and Others - Abstract - Adverse Reactions to Biologics - Karger Publishers [Internet]. [cited 2020 Sep 11]. Available from: https://www.karger.com/Article/Abstract/479475
- Generalized Pustular Psoriasis of Pregnancy (Impetigo herpetiformis) - Abstract - Dermatology 1999;198(1) - Karger Publishers [Internet]. [cited 2020 Sep 10]. Available from: https://www.karger.com/Article/Abstract/18066
- Novel findings of Langerhans cells and interleukin-17 expression in relation to the acrosyringium and pustule in palmoplantar pustulosis: Langerhans cells and IL-17 expression in PPP. Br J Dermatol. 2010;163:572-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acrosyringium Is the Main Site of the Vesicle/Pustule Formation in Palmoplantar Pustulosis. J Invest Dermatol. 2010;130:2010-6.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of nicotinic receptors in the skin of patients with palmoplantar pustulosis. Br J Dermatol. 2002;146:383-91.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of various types of therapy in patients with treatment-resistant acrodermatitis continua of Hallopeau. Biol Targets Ther. 2019;13:83-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis Auckl NZ. 2017;7:51-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- M S, Dm AA, Js C, S K. Pustular Psoriasis. 2019 Feb 7 [cited 2020 Sep 11]; Available from: https://europepmc.org/article/NBK/NBK537002
- Acute generalized exanthematous pustulosis with multisystem manifestations: Pinpoint pustules and purulent lakes. Ann Allergy Asthma Immunol. 2018;120:92-4.
- [CrossRef] [PubMed] [Google Scholar]
- An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907-19.
- [CrossRef] [PubMed] [Google Scholar]
- Generalized Pustular Psoriasis: Clinical Management and Update on Autoinflammatory Aspects. Am J Clin Dermatol. 2020;21:227-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Generalised pustular psoriasis and neutrophilic cholangitis: An infrequently reported association with excellent response to tumour necrosis factor inhibitors. Australas J Dermatol. 2017;58:70-1.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile generalized pustular psoriasis: Letters to the editor. Acta Derm Venereol 1998;78:220.
- Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis Targets Ther. 2016;6:131-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impetigo Herpetiformis with Lowered Serum Level of Vitamin D and Its Diminished Intestinal Absorption. Dermatology. 1982;164:360-5.
- [CrossRef] [PubMed] [Google Scholar]
- Impetigo herpetiformis: A variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41.
- [CrossRef] [PubMed] [Google Scholar]
- Impetigo herpetiformis and pustular psoriasis during pregnancy. Am J Dermatopathol. 1983;5:215-20.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of Acrodermatitis Continua of Hallopeau in Korea. Korean J Dermatol. 2018;56:106-13.
- [Google Scholar]
- Parakeratosis pustulosa: A diagnostic conundrum. Indian J Paediatr Dermatol. 2014;15:12.
- [Google Scholar]
- The role of psychological factors in palmoplantar pustulosis. J Eur Acad Dermatol Venereol. 2002;16:325-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study: Comparison of palmoplantar plaque psoriasis and PPP. Br J Dermatol. 2013;168:1243-51.
- [CrossRef] [PubMed] [Google Scholar]
- Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996–2013. Int J Dermatol. 2017;56:405-14.
- [CrossRef] [PubMed] [Google Scholar]
- Acute generalized exanthematous pustulosis (AGEP) – A clinical reaction pattern - Sidoroff - 2001 - Journal of Cutaneous Pathology - Wiley Online Library [Internet]. [cited 2020 Sep 11]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1034/j.1600-0560.2001.028003113.x
- Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295:S43-54.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pustular psoriasis: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-88.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical disease measures in generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(Suppl 1):39-50. https://doi.org/10.1007/s40257-021-00653-0
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 2018;45:1235-70.
- [CrossRef] [PubMed] [Google Scholar]
- Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis Auckl NZ. 2016;6:131-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Methotrexate in Moderate to Severe Psoriasis: Review of the Literature and Expert Recommendations. Actas Dermo-Sifiliográficas Engl Ed. 2016;107:194-206. Actas Dermo-Sifiliogr
- [CrossRef] [PubMed] [Google Scholar]
- Review of treatments for generalized pustular psoriasis. J Dermatol Treat. 2019;0:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Anti IL-17 in psoriasis. Expert Rev Clin Immunol. 2019;15:1185-94.
- [CrossRef] [PubMed] [Google Scholar]
- Generalized pustular psoriasis treated with apremilast in a patient with multiple medical comorbidities. JAAD Case Rep. 2017;3:495-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of two patients with generalized pustular psoriasis with the interleukin-1β inhibitor gevokizumab. Br J Dermatol. 2015;173:239-41.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pustular psoriasis with anakinra: a statistical analysis plan for stage 1 of an adaptive two-staged randomised placebo-controlled trial. Trials. 2018;19:534.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Granulocyte and Monocyte Adsorption Apheresis for Generalized Pustular Psoriasis: Therapeutic Outcomes in Three Refractory Patients. Ther Apher Dial Off Peer-Rev J Int Soc Apher Jpn Soc Apher Jpn Soc Dial Ther. 2015;19:336-41.
- [CrossRef] [PubMed] [Google Scholar]
- Impetigo Herpetiformis Complicated with Intrauterine Growth Restriction Treated Successfully with Granulocyte and Monocyte Apheresis. Acta Derm Venereol. 2017;97:410-1.
- [CrossRef] [PubMed] [Google Scholar]
- Small Molecule IL-36γ Antagonist as a Novel Therapeutic Approach for Plaque Psoriasis. Sci Rep. 2019;9:9089.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trial of Spesolimab for Generalized Pustular Psoriasis. NEJM 2021. N Engl J Med. 2021;385:2431-40.
- [CrossRef] [PubMed] [Google Scholar]
- ClinicalTrials.gov A Study to Evaluate the Efficacy and Safety of ANB019 in Subjects with Generalized Pustular Psoriasis (GPP) [(accessed on 15 May 2021)];NCT03619902. Available online: https://clinicaltrials.gov/ct2/show/NCT03619902
- The Efficacy of Biologic Therapy for the Management of Palmoplantar Psoriasis and Palmoplantar Pustulosis: A Systematic Review. Dermatol Ther. 2017;7:425-46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pustular psoriasis–Clinical study in a tertiary care center. Journal of Dr. NTR University of Health Sciences.. 2018;7:259.
- [Google Scholar]
- Measuring quality of life of patients with different clinical types of psoriasis using the SF-36. Br J Dermatol. 2006;154:844-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: ANALYSIS of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-84.
- [CrossRef] [PubMed] [Google Scholar]
- Economic Burden of Generalized Pustular Psoriasis in Sweden: A Population-Based Register Study. Psoriasis (Auckl).. 2022;12:89-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






