Translate this page into:
Pyoderma gangrenosum following BCG vaccination
Corresponding author: Dr.G.Raghurama Rao, Consultant Dermatologist, Surya Skin Care Center, Visakhapatnam. graghuramarao@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Haritha K, Raghu GRR, Sudhir KB. Pyoderma gangrenosum following BCG vaccination. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1515_2024
Dear Editor,
Although the Bacillus Calmette-Guérin (BCG) vaccine is usually safe and well tolerated, complications, such as adverse local reactions, regional lymphadenitis, osteomyelitis, and disseminated BCGosis can occur in immuno-compromised children.1 We report a case of pyoderma gangrenosum following BCG vaccination as an interesting association.
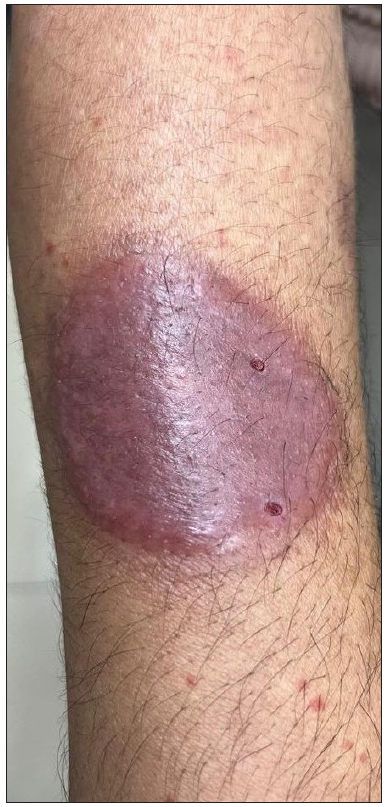
A 55-year-old healthy man underwent BCG vaccination as a part of the adult BCG vaccination pilot project. After 2-3 days he developed a small papule at the site of vaccination (right arm), which healed with crust formation. After 20 days, he developed papulo-pustular lesions on the dorsal aspect of the right forearm, and these lesions formed an annular plaque. The lesion was progressive and attained the size of 7×5 cm by the time of examination. The lesion was a tender, annular plaque with well-defined margins studded with multiple pustules and the center was scaly and crusted [Figures 1a and 1b]. There was no past history of BCG vaccination or any systemic illnesses. He had no constitutional symptoms and no regional lymphadenopathy. Systemic examination revealed no abnormalities. All relevant hematological, biochemical, and serological tests were within normal limits. Chest X-ray and abdomen ultrasonography were done to rule out other systemic illnesses which were normal. Edge biopsy of the plaque showed marked subepidermal edema with dense neutrophilic infiltration along with a few eosinophils in the superficial dermis. In the deep dermis, there was a mild perivascular inflammatory infiltrate composed of neutrophils, lymphocytes, and a few eosinophils. There was no evidence of vasculitis [Figures 2a and 2b]. Special stains like Periodic Acid-Schiff (PAS), Ziehl Neelsen (ZN) stain, and gram stain were negative. These findings were suggestive of neutrophilic dermatoses and we considered the following as differentials: Sweet’s syndrome, evolving pyoderma gangrenosum (PG), and neutrophilic dermatoses of the dorsal hand. As our patient was asymptomatic and afebrile, and had no systemic illnesses, apart from cutaneous lesions we were in favour of the vegetative type of PG. It is highly likely BCG vaccination could have contributed to the development of PG due to their close temporal relationship. We treated the patient with oral prednisolone 40 mg daily, which resulted in dramatic improvement [Figure 3]. The treatment was tapered off and stopped within 4 weeks without recurrence of the eruption.
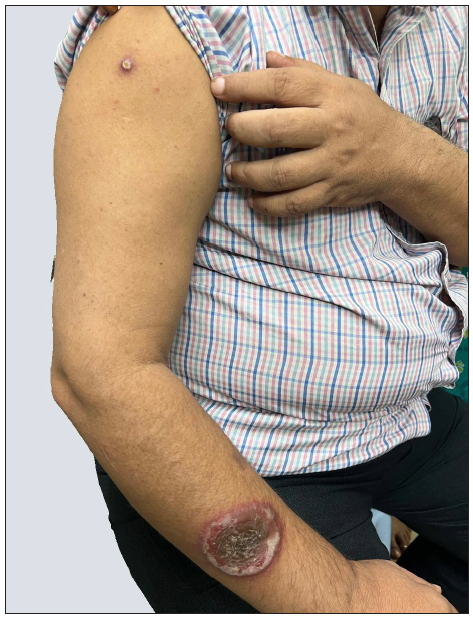
- BCG vaccination site (right deltoid area) with pyoderma gangrenosum (right forearm).
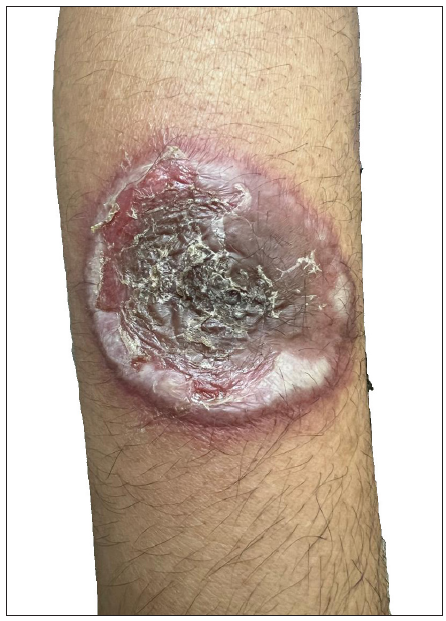
- Close-up view of the pyoderma gangrenosum lesion on the forearm.
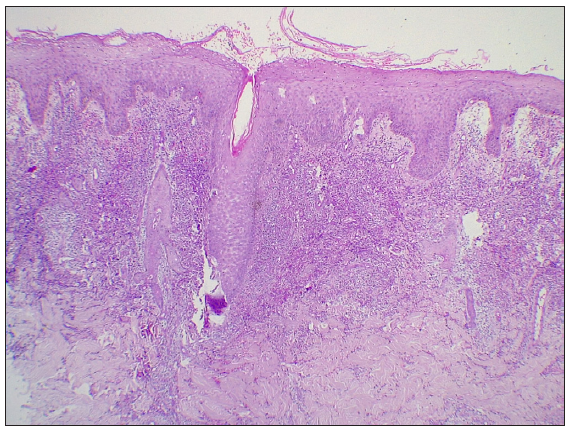
- Intact epidermis with sub-epidermal edema. Dermis shows dense neutrophilic infiltrate with few eosinophils and mild perivascular inflammatory infiltrate. (Haematoxylin & eosin, 100x).
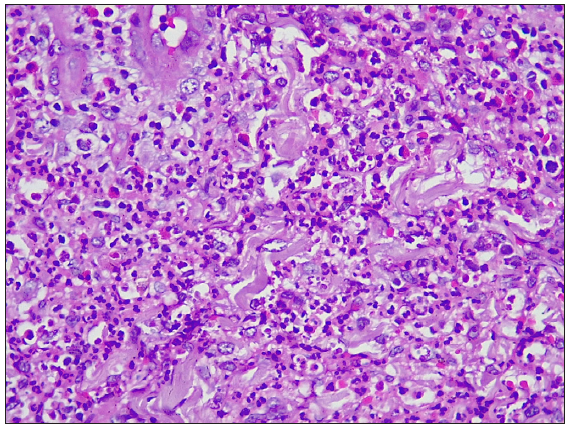
- Dense neutrophilic infiltrate with few eosinophils (Haematoxylin & eosin, 400x)
- Post-treatment image
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatoses characterised by a painful, aseptic ulcer with tissue destruction. In half of the cases, PG is associated with numerous systemic diseases like autoimmune diseases, inflammatory bowel disease, and malignancies. Various infectious agents, vaccines, and drugs act as triggers. The pathogenesis of PG is complex and unclear. In a genetically predisposed person, a cascade of inflammatory events, including activation of innate immunity, auto-inflammatory pathways, neutrophil dissemination, recruitment of adaptive immune response, over activation of numerous cytokines, interleukins (IL-8, IL-17, IL-23, TNF) can result in tissue destruction.2 Cutaneous lesions usually begin as pustules or vesiculo-pustules and progress to ulcers or depressions. Apart from the classic ulcerative PG; peristomal, pustular, bullous, and vegetative types have been reported. The vegetative variant is superficial and less aggressive than other types of PG. Vegetative PG usually occur as a solitary lesion, progresses slowly, and is more responsive to treatment. Diagnosis of PG is mainly clinical, done by excluding other neutrophilic dermatoses and causes of cutaneous ulceration. There are no established specific histological criteria to diagnose PG. Three diagnostic criteria (Su, Maverakis plus International Delphi criteria and Paracelsus criteria) have been proposed to aid in the recognition of PG and these three criteria were thoroughly discussed by Skopis et.al.2 Currently, Paracelsus criteria was the most successful diagnostic tool in PG.3
The association between tuberculosis and PG has been recently reported.4 The relationship between tuberculosis and neutrophilic dermatoses is not fully known, but tuberculosis infection is known to trigger system-wide inflammation, which could potentially lead to PG. There were reports of sweet syndrome following BCG vaccination.5,6 But to our knowledge there has been no case of PG at distant site post-BCG vaccination. Our case shows an interesting association of PG following BCG vaccination. BCG is a live bacterial vaccine containing the Danish 1331 strain of M.bovis used in India. The vaccine contains about 0.1-0.4 million live viable bacilli per dose. An amount of 0.05ml of reconstituted vaccine is given in the lateral aspect of the left arm intradermally. In this case, the pathogenesis is unclear. As PG is thought to be an abnormal immunological activity with hypersensitivity to various infectious antigens, circulating mycobacterium antigens (live vaccine) might have triggered the cutaneous inflammation. Recently, Radeff et.al and Toyama et.al reported cases of PG induced by COVID-19 vaccines.7,8 The authors speculated that Th1/Th17 mediated cutaneous inflammation was induced by COVID-19 vaccination, leading to the development of PG. The purpose of reporting this rare complication of BCG vaccination is to create awareness among clinicians to help them timely recognise it.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Disseminated BCG vaccination in immunocompomised child: A case report. Pediatr Inf Dis. 2023;5:25-6.
- [Google Scholar]
- Pyoderma gangrenosum: A review of updates in diagnosis, pathophysiology and management. J. 2021;4:367-75.
- [Google Scholar]
- Comparison of three diagnostic frameworks for pyoderma gangrenosum. J Investig Dermatol. 2021;141:59-63.
- [CrossRef] [PubMed] [Google Scholar]
- Concurrent pyoderma gangrenosum and tuberculosis: Diagnosis, treatment complexities and exploring cyclosporine’s role - A literature review. Indian J Dermatol Venereol Leprol 2024:1-4.
- [Google Scholar]
- Acute febrile neutrophilic dermatosis (Sweet’s syndrome) following BCG vaccination. Acta Derm Venereol. 1986;66:357-8.
- [PubMed] [Google Scholar]
- Sweet’s syndrome after BCG vaccination. Acta Derm Venereol. 2002;82:221.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum induced by BNT162b2 COVID-19 vaccine in a healthy adult. Vaccines (Basel). 2022;10:87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pyoderma gangrenosum following vaccination against coronavirus disease-2019: A case report. Int J Dermatol. 2022;61:905-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





