Translate this page into:
Rapid progression of cutaneous T-cell lymphoma in a patient with erythroderma during dupilumab treatment, following prior sequential azathioprine, baricitinib and cyclosporine treatments
Corresponding author: Dr. Tsen-Fang Tsai, Department of Dermatology, National Taiwan University Hospital, No.7, Chung Shan S. Rd. Zhongshan S. Rd, Zhongzheng Dist., Taipei City, Taiwan tftsai@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Hsieh C-Y, Tsai T-F. Rapid progression of cutaneous T-cell lymphoma in a patient with erythroderma during dupilumab treatment, following prior sequential azathioprine, baricitinib and cyclosporine treatments. Indian J Dermatol Venereol Leprol. 2024;90:353-5. doi: 10.25259/IJDVL_1090_2022
Dear Editor,
Dupilumab is, in general, a well-tolerated medication indicated for the treatment of patients with atopic dermatitis. However, reports of progression or development of cutaneous T-cell lymphoma exist.1 Here, we report a patient diagnosed with erythrodermic atopic dermatitis who had rapid progression of mycosis fungoides with CD30+ large cell transformation during dupilumab treatment, following prior sequential treatment with azathioprine, baricitinib and cyclosporine.
A 34-year-old man with a family history of atopic diathesis presented to our clinic in 2017 with generalised itching, eczematous plaques and ichthyosis vulgaris since the age of eight [Figures 1a and 1b]. With suspicion of erythroderma due to atopic dermatitis, a biopsy was performed which showed psoriasiform epidermal hyperplasia with mild spongiosis, parakeratosis and small foci of Langerhans cell microgranulomas suggestive of spongiotic dermatitis. There was moderately dense band-like lymphocytic infiltration in the superficial dermis with scattered eosinophils. However, moderate lymphocyte exocytosis with focal nuclear atypia was present [Figure 2a].
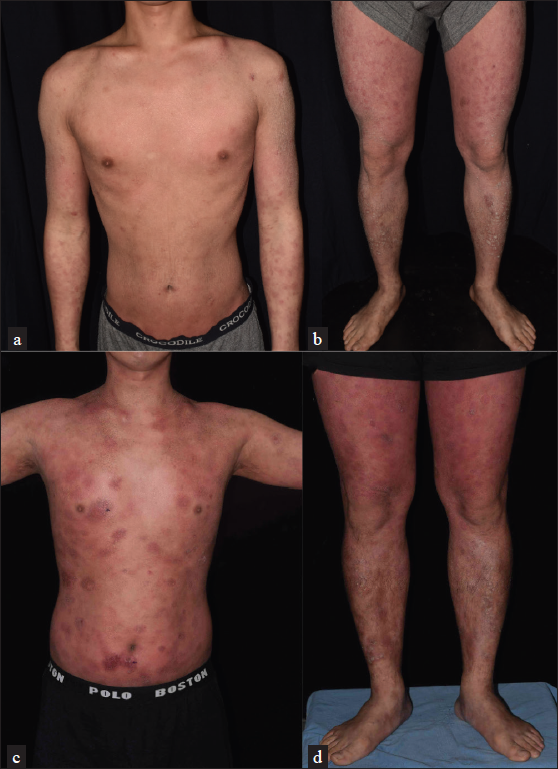
- (a and b) There were generalised erythematous, lichenified, excoriated plaques with brownish adherent scales on the legs. (c and d) After treatment with dupilumab, the skin lesions worsened. There were generalised, thick, erythematous, infiltrative plaques
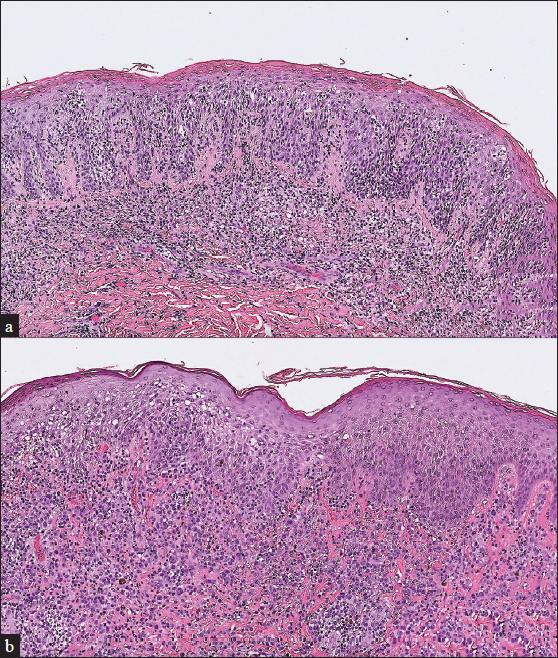
- (a) Initial biopsy showed psoriasiform epidermal hyperplasia with mild spongiosis, parakeratosis, and small foci of Langerhans cell microgranuloma. Moderate lymphocyte exocytosis with focal nuclear atypia was also seen (H&E, 100x). (b) Repeat biopsy showed proliferation of sheets of large atypical lymphoid cells in the dermis with acanthosis (H&E, 100x)
The patient had failed various topical treatments and refused phototherapy. Due to concern of methotrexate toxicity, he was initially treated with azathioprine 100 mg daily in 2017 for 10 months in our clinic. Due to inadequate response, it was replaced with baricitinib 4 mg daily from January to August in 2018. Since April 2019, he is taking treatment from another hospital with cyclosporine 100–150 mg daily. Because of lack of improvement, the treatment drug was changed to dupilumab since January 2020. Nonetheless, gradual thickening of some pre-existing papules and plaques was noted after the drug switch [Figures 1c and 1d]. Skin biopsy was repeated in April 2020, and CD8+ mycosis fungoides was diagnosed based on the features of a lichenoid infiltrate of lymphocytes, histiocytes and eosinophils with epidermotropism. The lymphocytes were positive for CD3 and CD8, with a partial loss of CD7. Dupilumab was discontinued in April 2020, and he was treated with photochemotherapy, along with acitretin and methotrexate.
Despite the aforementioned treatments, biopsy performed in September 2020 revealed mycosis fungoides with large cell transformation [Figure 2b]. Upon examination, the patient had confluent erythema covering >80% of the body surface area. Whole body computer tomography showed axillary and inguinal lymphadenopathy, and biopsy revealed dermatopathic lymphadenopathy. Mycosis fungoides, T4N1M0B0, stage IIIA was confirmed. Chemotherapy with cyclophosphamide, etoposide, vincristine and prednisone (CEOP) was started since December 2020, which resulted in regression of axillary and inguinal lymphadenopathy. However, persistent scattered, infiltrative, erythematous plaques were noticed. Another biopsy in April 2022 revealed mycosis fungoides with CD30 expression in >80% of neoplastic cells. Thus, brentuximab vedotin was introduced since May 2022, and complete metabolic remission on 18F-FDG-PET scan was achieved in August 2022 without relapse until now.
Mycosis fungoides may mimic eczema, and an early diagnosis can be challenging. Usually, atopic dermatitis first appears in childhood, while mycosis fungoides has a much later onset. The diagnosis may be especially difficult in patients who present with erythrodermic mycosis fungoides and have an atopic diathesis. In our patient, an equivocal pathologic finding with atypical lymphocytes favouring dermatitis was reported. Among the oral agents used for the treatment of atopic dermatitis, methotrexate is also used for the treatment of cutaneous T-cell lymphoma. In contrast, increased risk of T-cell lymphoma was observed in patients with inflammatory bowel diseases on azathioprine,2 but no cases of cutaneous T-cell lymphoma have been reported in patients with atopic dermatitis, who were treated with either azathioprine or methotrexate.
The risk of lymphoma in patients with dupilumab has not been investigated in large-scale studies. Kołkowski et al. recently investigated the safety profiles of novel treatments for atopic dermatitis in the context of cutaneous T-cell lymphoma, and risks were observed only in patients treated with dupilumab and ruxolitinib, but not with other Janus kinase inhibitors such as baricitinib, upadacitinib and abrocitinib.3
The risk of cutaneous T-cell lymphoma in patients treated with dupilumab could be explained by several hypotheses. Dupilumab inhibits both IL-4 and IL-13 by targeting IL-4 receptor α (IL-4Rα). Blocking the IL-4Rα may paradoxically stimulate IL-13 signaling pathway via IL-13 receptor α2. IL-13 is associated with the pathogenesis and progression of various malignancies, including cutaneous T-cell lymphoma proliferation.4
It is worth noting that our patient had a long disease course since childhood, and his skin condition was either stable or was slightly improved from various agents that were considered to be “immunosuppressive,” including azathioprine, cyclosporine and baricitinib. Although dupilumab is considered a non-immunosuppressive agent, there was a rapid worsening of skin lesions after its introduction in 2020. Given the time sequence, rapid worsening of skin lesions after treatment and prior reports, dupilumab might have played an important role in the development of cutaneous T-cell lymphoma in the patient. However, methotrexate, azathioprine and cyclosporine were also related to the development of lymphoma or increased risk of lymphoma in patients with inflammatory bowel disease.5 Thus, the role of his preceding treatments, especially cyclosporine, in the development or progression of lymphoma cannot be totally excluded.
In our patient, it is likely that an early onset of erythrodermic mycosis fungoides was present as evidenced by the lack of adequate response to all systemic treatment of atopic dermatitis. In patients with erythroderma whose skin biopsy showed spongiotic dermatitis with suspicious atypical lymphocyte exocytosis, the use of systemic immunomodulators including dupilumab should be done with caution. Dermatologists should be familiar with the possibility of cutaneous T-cell lymphoma initiation or progression during the treatment of atopic dermatitis, especially in patients presenting with a sudden deterioration of existing lesions or the development of new eczematous plaques or lymphadenopathy. Further studies are warranted to investigate the possible immunopathogenic association between dupilumab and cutaneous T-cell lymphoma development.
Acknowledgement
We appreciated Dr. Yu-Chien Ko’s help for capturing the pathological slides.
Authors contributions
In this manuscript, Chang-Yu Hsieh contributed to the literature search, clinical studies, data acquisition, data and statistical analysis and manuscript preparation. Tsen-Fang Tsai contributed to the concepts, design, definition of intellectual content, experimental studies, and manuscript editing and review.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Progression of cutaneous T-cell lymphoma after dupilumab: Case review of 7 patients. J Am Acad Dermatol. 2020;83:197-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- T-cell non-Hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-α) inhibitors: Results of the REFURBISH study. Am J Gastroenterol. 2013;108:99-105.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and danger considerations of novel treatments for atopic dermatitis in context of primary cutaneous lymphomas. Int J Mol Sci. 2021;22:13388.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comment on “Diagnostic testing of eczematous dermatitis with incomplete response to dupilumab”. J Am Acad Dermatol. 2022;87:e241-2.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the risk of lymphoma following immunosuppressive therapy for inflammatory bowel disease. Exp Rev Clin Immunol. 2010;6:621-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






