Translate this page into:
Recalcitrant chronic actinic dermatitis responding to tofacitinib: A case report
Corresponding author: Dr. Keshavamurthy Vinay, Associate Professor, Department of Dermatology Venereology & Leprology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dev A, Bishnoi A, Narang T, Vinay K. Recalcitrant chronic actinic dermatitis responding to tofacitinib: A case report. Indian J Dermatol Venereol Leprol 2023;89:600–2.
Dear Editor,
Chronic actinic dermatitis is an idiopathic photosensitive dermatitis. We hereby describe a patient with chronic actinic dermatitis who was refractory to the conventional therapies but responded well to oral tofacitinib, a Janus kinase inhibitor.
A 50-year-old man, a businessman by occupation, presented with itchy and mildly scaly plaques involving the face, scalp, upper chest, back and extensors of upper and lower limbs. He reported exacerbation of redness of the lesions as well an increase in pruritus and burning sensation on exposure to sunlight. There was a history of hair dye usage but no history of atopy or exposure to parthenium at his place of residence or work. Patch test and photopatch test could not be performed as he was not disease-free at any point in time. He was a known diabetic and hypertensive, which was being managed with metformin, glimepiride, telmisartan and amlodipine. Dermatitis severely affected his quality of life with a baseline Dermatology Life Quality Index of 14 and he rated pruritus 9 on the visual analogue scale. General and systemic examination was unremarkable. Cutaneous examination revealed photodistributed, erythematous and eczematous plaques over the face [Figure 1a], neck, helices of the ears, scalp, V area of the chest, upper back, extensors of the upper limb and lower limb and dorsum of hands and feet, with lichenification at places. There was notable sparing of the upper eyelids, submental area, retroauricular area and nasolabial folds, as well as photoprotected areas. The Eczema Area and Severity Index score at baseline was 15.8.
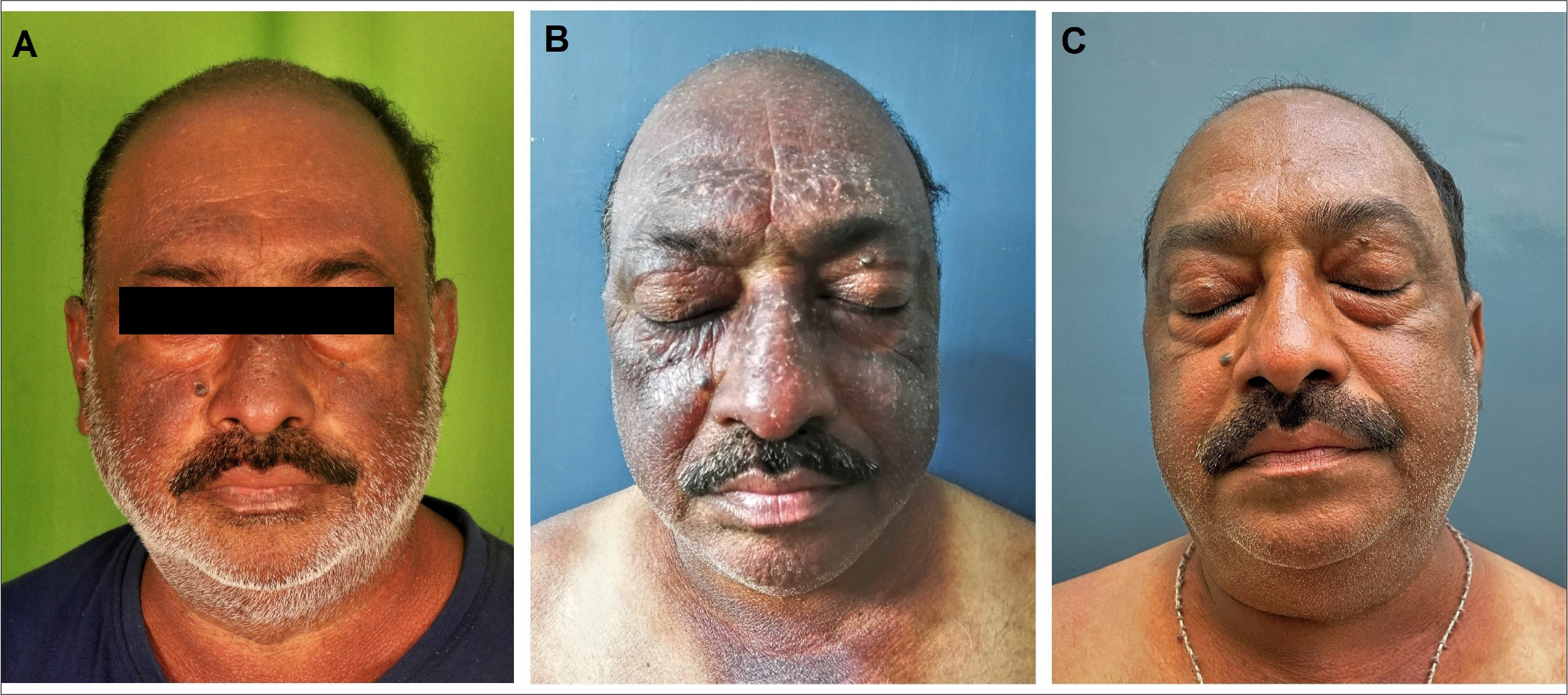
- (A) Index case of chronic actinic dermatitis showing eczematous plaques over forehead, malar area, ear helix and V area of neck. The upper eyelid and nasolabial fold are characteristically spared. (B) Clinical presentation prior to starting tofacitinib therapy, shows exacerbation with increased erythema and scaling over the face. (C) Six weeks post-tofacitinib there has been significant improvement in erythema, scaling and lichenification.
Routine blood investigations like complete blood count, liver and renal function tests were normal. No atypical cells were visualised on the peripheral smear. Anti-nuclear antibody by indirect immunofluorescence was positive (1+, speckled pattern). Histopathological examination of the lesions from the dorsum of the hand revealed parakeratosis, irregular acanthosis, few apoptotic keratinocytes and focal basal cell vacuolisation. A lymphocytic infiltrate was seen in the papillary dermis. Based on these findings, a diagnosis of chronic actinic dermatitis was made. Glimepiride was stopped in view of its photosensitising action and he was started on alternate anti-diabetic medications (vildagliptin and dapagliflozin).
Initially, the patient was advised of potent topical corticosteroids (clobetasol propionate), along with topical tacrolimus, oral hydroxyzine, broad-spectrum sunscreen and physical photoprotective measures. However, the response was unsatisfactory. Over the course of the subsequent year, he was managed with tapering doses of oral prednisolone during flare-ups, oral methotrexate, azathioprine, acitretin and hydroxychloroquine at conventional doses over 3–6 months with minimal response [Figure 1b]. He was then started on oral tofacitinib monotherapy after ruling out hepatitis B and C, human immunodeficiency virus and tuberculosis (chest X-ray and Mantoux test) and a normal fasting lipid profile at a dose of 5 mg twice a day. About six weeks after initiation of tofacitinib, the patient reported significant improvement in pruritus, burning sensation and infiltration of lesions [Figure 1c]. The response was maintained and after three months of therapy, there was a near-complete clearing of lesions at all sites (post-treatment Eczema Area and Severity Index score = 0.9) along with the complete resolution of symptoms (visual analogue scale score for pruritus = 0). Regular monitoring of complete blood counts, liver function tests and fasting lipid profile was performed and no adverse effects were observed. There was also a dramatic improvement in his quality of life and his Dermatology Life Quality Index post-treatment was calculated to be 2. The patient is currently under follow-up and tofacitinib has been tapered to 5 mg once a day after 6 months.
Chronic actinic dermatitis is a photosensitive eczematous dermatosis1 that commonly affects middle-aged and elderly men. 2 Management involves strict photoprotection, which comprises sunscreens and physical measures like long-sleeved clothing, seeking shade during peak hours and covering windows. 3 Middle to high potent topical corticosteroids and tacrolimus are used as first-line therapies. 3 More extensive disease, however, warrants the use of oral immunosuppressants like oral corticosteroids, azathioprine, methotrexate and mycophenolate mofetil. 1 Despite the availability of multiple therapeutic options, chronic actinic dermatitis can be persistent and refractory to treatment.
Tofacitinib is an oral Janus kinase 1/3 inhibitor that is increasingly being used in multiple dermatoses like psoriasis, vitiligo, alopecia areata, dermatomyositis, etc. The exact pathogenesis of chronic actinic dermatitis remains elusive; however, a delayed-type hypersensitivity mediated by T cells to a photoinduced cutaneous endo-antigen is proposed. 1 The excellent response of chronic actinic dermatitis to tofacitinib in our patient suggests a role of Janus Kinase-Signal transducer and activators of transcription (JAK-STAT) inhibition in the suppression of T cell-mediated diseases, including eczematous dermatoses. Imran Majid and Saniya Akhtar 4 and Vesely et al. 5 have also reported excellent responses of long-standing and refractory chronic actinic dermatitis to tofacitinib [Table 1], with significant improvement in quality of life, as seen in our patient as well. Although our patient did not experience any adverse effects, bone marrow suppression, derangement of liver function tests, hyperlipidaemia, reactivation of infections, etc. have been known to occur with tofacitinib and both baseline investigations and regular monitoring must be undertaken.
| Authors | Type of report | Prior medications received | Dose of tofacitinib | Response | Adverse effects |
|---|---|---|---|---|---|
| Imran Majid and Saniya Akhtar | Case series | Methotrexate, azathioprine, hydroxychloroquine, cyclosporine and apremilast | 5 mg twice a day, tapered to once daily after 6 months | Clearing of lesions within 4–8 weeks of initiation of therapy | None |
| Vesely et al. | Case report | Topical steroids, topical tacrolimus, oral prednisone, hydroxychloroquine, methotrexate, azathioprine, mycophenolate mofetil, cyclosporine, omalizumab, acitretin, oral bexarotene, extracorporeal photopheresis | 5 mg twice a day | Clearing of lesions within 8 weeks of initiation of therapy | Herpes zoster |
To conclude, oral tofacitinib can be considered a therapeutic option in extensive and recalcitrant chronic actinic dermatitis. Our case adds to the literature on the utility of JAK-STAT inhibitors in the treatment of chronic actinic dermatitis. Further controlled studies are needed to establish its efficacy in eczematous diseases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Diagnosis and treatment of chronic actinic dermatitis. Dermatol Ther. 2003;16:45-51.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib in resistant chronic actinic dermatitis: A case series. Dermatol Ther. 2022;35:e15467.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib citrate for the treatment of refractory, severe chronic actinic dermatitis. JAAD Case Rep. 2017;3:4-6.
- [CrossRef] [PubMed] [Google Scholar]





