Translate this page into:
Recent advances in the control programs and therapy of leprosy
Correspondence Address:
P Narasimha Rao
B-48, Income Tax Colony, Mehdipatnam, Hyderabad - 500 028
India
| How to cite this article: Rao P N. Recent advances in the control programs and therapy of leprosy. Indian J Dermatol Venereol Leprol 2004;70:269-276 |
Abstract
Leprosy control programs, including multi-drug therapy for leprosy, have undergone significant changes over the last few years. With the process of integration of leprosy into general health services taking place all over India, dermatologists are more responsible for the care of leprosy patients than ever before. This article attempts to highlight some of the important changes in control programs and advances in the therapy of leprosy.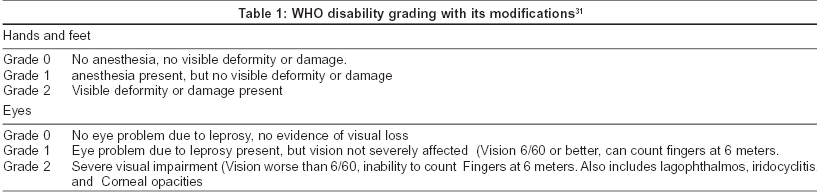

The most important development in the leprosy control in the last millennium has been the introduction of multi-drug therapy (MDT) in 1982, following the recommendation of the WHO study group.[1] There has been a dramatic downward revision of the estimated number of leprosy patients in the world from 10 to 12 million in the mid 1980s to 0.62 million (point prevalence) in 2001. Although these figures are highly encouraging, the number of new cases detected annually has remained quite stable during the last 15 years.[2] It is worthwhile to note that the number of new cases detected globally in 2001 (0.75 million) was more than the point prevalence of that year.
Of the 122 countries where leprosy was considered endemic in 1985, 110 have now reached the goal of elimination at the country level by 2003 and leprosy remains a public health problem only in 12 countries.[3] The burden of leprosy is concentrated in five most endemic countries, which are India, Brazil, Nepal, Madagascar, and Myanmar, in that order of importance. These countries account for 83% of the prevalence and 88% of new case detection worldwide. The combined prevalence rate of these countries is 4 per 10,000 populations.
INDIAN SCENARIO[4]
India harbors 65% of the world′s population of leprosy patients. The total number of registered cases in India shows a steep decline over the years, from 3.4 million cases in 1986 to 0.266 million cases in 2004. The leprosy prevalence rate has gone down from 50.3 per 10,000 in 1986 to 2.44 per 10,000 by March 2004. Twenty-one states and union territories in India have either eliminated leprosy or have only a few hundred cases each. However, there is considerable regional variation in progress in the elimination of leprosy in India. One fifth of India′s leprosy patients are in Bihar and more than one sixth are in Uttar Pradesh. Although much progress has been made towards reaching the goal of elimination of leprosy (which is defined as a prevalence of less than one case per 10,000 population), serious challenges remain in many parts of India. In fact, there was a 10% increase in the number of new cases detected in India in the year 2001 compared to 2000.[5]
The key to reach the goal of leprosy elimination in an endemic country is to diagnose and treat leprosy through the public health services. The WHO Expert Committee on Leprosy, for the first time in its seventh report of 1998,[6] mentions the need for assigning a role to dermatologists for the elimination of leprosy. It stresses the need to include leprosy as a part of the curriculum of dermatology and the need to encourage dermatologists to ensure that standard WHO MDT regimens are implemented and new cases are reported. This is one more reason for Indian dermatologists to keep track of recent developments and changes in the leprosy control programs and schedules as integration of leprosy into general health services has already begun.
Now we shall focus on changes in certain basic definitions and concepts of leprosy which influence therapy.
DEFINITION OF A CASE OF LEPROSY[6]
The WHO in 1998 defined a case of leprosy as a person having one or more of the following features and who has yet to complete a full course of treatment:
1. Hypopigmented or reddish skin lesion(s) with definite loss of sensation.
2. Involvement of peripheral nerves as demonstrated by definite thickening with loss of sensation.
3. Skin smear positive for acid-fast bacilli (AFB).
This definition also includes treatment defaulters and cases who relapse, but does not include cured persons or persons with late reactions or residual disabilities.
However, not all workers agree with the above definition of leprosy. Although the simplification of guidelines for diagnosis is made for the benefit of non-specialists who will be managing leprosy after integration, it is felt that applying these definitions will lead to underdiagnosis, particularly of multibacillary (MB) disease.[7] Approximately 70% of leprosy patients can be diagnosed by means of a single sign of skin patch with sensory loss. However, 30% of patients, including many MB patients, do not present with this sign. The technical forum of International Leprosy Association in its report of 2002 concludes that at least two of the traditional cardinal signs are necessary to achieve a reasonable degree of sensitivity in the diagnosis of leprosy, as using anesthetic patches as the only sign of leprosy is inadequate. It also states that one or more enlarged nerve is an acceptable additional sign, to be supplemented by skin smears when available.
STATUS OF SKIN SMEARS
Skin smears have traditionally represented one of the cardinal signs of leprosy. When positive, skin smears directly demonstrate the presence of M. leprae and thus confirm the diagnosis. In experienced hands, the specificity of the examination approaches 100%. However, the sensitivity of the smears alone is low, because smear positive patients rarely represent more than 50% and sometimes as few as 10% of all patients of leprosy.[7] The WHO has made it clear that skin smears are not a prerequisite for managing leprosy elimination programs and that there is no need to maintain or establish skin smear services exclusively for leprosy.[3] Although skin smears are useful in diagnosing multibacillary leprosy, the quality of skin smears and microscopy is probably the weakest link in most leprosy programs. The other factors which influence this decision was that fewer than 15% of newly diagnosed cases show positive results and the diagnosis is rarely based on skin smear results under field conditions. Moreover, all skin-piercing procedures carry the potential risk of transmitting HIV and hepatitis infections. The WHO opines that use of skin smears should be limited to referral centers and particularly for special investigations such as suspicion of resistance and for research purposes.
CLASSIFICATION OF LEPROSY FOR TREATMENT PURPOSES[6]
The WHO proposed that the clinical system of classification of leprosy for treatment purposes should include the number of skin lesions as a basis for classifying leprosy patients into multibacillary (MB) and paucibacillary (PB) groups. It proposed that patients with 2-5 skin lesions should be considered as PB for treatment purposes and those with more than 5 skin lesions should be considered as MB for treatment purposes. It also had a special status for single-lesion leprosy patients.
Single lesion PB leprosy (SLL-PB) refers to those patients who have only one hypopigmented or reddish skin lesion with definite loss of sensations but without nerve trunk involvement. The specificity of the diagnosis of SLL-PB remains uncertain as various operational factors affect the specificity of the diagnosis. In general, SLL-PB accounts for a significant proportion of newly diagnosed cases ranging from 20-30% in Malawi to close to 60% in India.
The SLL-PB group is important for purposes of therapy as the WHO recommended a single dose drug combination of rifampicin 600 mg, ofloxacin 400 mg, and minocycline 100 mg (ROM) for its treatment. This recommendation was based on a multicentric double blind field trial conducted in India.[8] Although the single dose of ROM was marginally less effective in terms of clinical improvement than the standard MDT regimen, the committee considered that a single dose of ROM is an acceptable and cost effective alternative regimen for the treatment of patients belonging to this category. The Directorate General of Leprosy, Government of India, had accepted the ROM regimen and had incorporated it in its National Leprosy Eradication Programme (NLEP) in November 1997.[9] However, they have recommended that patients on ROM should be under surveillance for 2 years after administration of therapy. A WHO sponsored multi-centre clinical trial coordinated by the National Institute of Epidemiology at Chennai, India is currently evaluating the efficacy of single dose ROM treatment for skin smear negative PB leprosy cases compared to the standard 6-month PB MDT regimen. The study is now in its 4th year and detailed results will be available only by 2005.[10]
However, it is important to note that the WHO in its recent communications of 2003 has omitted any separate mention of the SLL-PB group or ROM therapy.[3],[11] WHO websites on leprosy presently classify leprosy for treatment purposes into PB leprosy (patients with 1-5 skin lesions) and MB leprosy (patients with more than 5 skin lesions). The WHO at present is pursuing newer strategies such as Uniform-MDT and Accompanied-MDT vigorously, and it appears that ROM therapy is not a priority, at least for the time being.
MULTI-DRUG THERAPY AND ITS MODIFICATIONS
The standard schedule of MDT for leprosy has not undergone any change after its introduction in 1982, except for the temporary introduction of ROM therapy for single skin lesion-PB leprosy. However, there have been significant changes in the duration of the therapy and in the criteria for allocating patients into PB and MB groups for therapy. At the time of introduction of MDT, in 1982, this allocation was based on the grading of smear positivity, with patients with a bacteriological index (BI) of < 2 classified as PB leprosy. In 1988, the WHO Expert Committee on Leprosy recommended that only initially smear negative patients should be classified as PB.[12] With time, a division based on skin smear examination was not considered suitable in field conditions and a division based on the number of skin lesions gained favor. In the last 22 years, an increasing number of patients who were previously classified as PB for treatment purposes were allocated to the MB group for treatment purposes, with skin smear examination no longer considered necessary for grouping. Patients with more than 5 skin lesions are now being classified as MB patients,[6] and wherever facilities are available for a slit skin smear, all smear positive patients are included in the MB group.
The original recommendation of treating patients till smear negativity meant that the duration of treatment was for more than 2 years for MB patients. This was reduced to a fixed duration of 24 months in 1992,[13] and further shortened to 12 months in 1998.[6] The duration was reduced to 12 months based on the following justifications detailed in the Seventh WHO Expert Committee report:
a) Experimental studies in animals indicating that the bactericidal effect of clofazimine and dapsone as companion drugs to rifampicin was greater than what was estimated earlier and was capable of eliminating any rifampicin resistant mutants in an untreated MB patient in about 3 to 6 months.
b) Several studies demonstrated that MB leprosy patients given only a few monthly doses of MDT responded as favorably as those who received 24 or more doses.
c) Limited controlled trials indicated that a fixed 12-month MDT was effective in preventing relapse even after 4 years of follow-up.
At present 12 months′ fixed duration therapy for MB patients is being followed all over India.
UNIFORM MDT
Increasing confidence in the effectiveness of 12-month MDT for MB patients has led to attempts to further shorten it to six months. A WHO technical advisory group, in its third meeting in 2002,[14] proposed that a uniform MDT regimen (U-MDT) should be considered to treat all types of leprosy. The group felt that with MDT being widely implemented with very low relapse rates and complete absence of emergence of M. leprae resistance, further shortening and simplification of the MDT regimen by introducing uniform MDT would lead to easier logistics support, simpler information system, reduced training needs and thus better sustainability through integration. However, while there is some favorable evidence from animal studies and from some field observations of defaulting patients, there is yet no evidence from any controlled clinical trials, even of a limited nature, to support this shortening of duration of the treatment. The proposal was to develop a protocol after studying the results of a large-scale field trial of MB MDT regimen for 6 months as a uniform regimen for both PB and MB patients.
However, the WHO technical committee in its 5th meeting in 2003[11] opined that such a trial was not feasible, and hence not necessary. It has mentioned a few reasons why such a randomized controlled trial cannot be considered. The main issue to be addressed for this group is one of acceptability and it was felt that it could be tackled in an open study design. If a randomized controlled trial needs to be conducted at all, it could be justified in the small fraction of patients with high BI. However, as the sample size calculations have to be based on the principle of equivalence, the numbers would be enormously large and hence such a trial is not a practical proposition.
Expectedly, there has been strong criticism for this proposal to introduce U-MDT for all patients of leprosy.[15] Many felt that U-MDT will over-treat PB leprosy patients and under-treat MB patients, especially those with a high initial BI. This recommendation is yet to be implemented in India.
Accompanied MDT or A-MDT is designed by the WHO to address frequent problems in the field programs. A-MDT means providing patients with a full course of treatment on their first visit to the leprosy clinic after diagnosis. On this occasion the patient and the accompanying person also receive information about leprosy in the form of printed material about the disease, its treatment and when and where to come for follow-up or in the event of complications. The term accompanied is adopted because someone close to or important to the patient assumes responsibility for helping the patient to complete the treatment. The WHO feels that A-MDT is user friendly to mobile populations, patients living in remote areas and in areas of civil strife. It also hopes that this method will increase patient compliance and decrease default.
Notwithstanding WHO′s reasoning, A-MDT is controversial because it deviates fundamentally from the principle of supervised administration of the monthly component of the standard MDT regimen.[6] Because the monthly supervised component is no longer administered, the frequency of contact between the patient and the health worker would be reduced, which would affect the regularity of drug administration. Moreover, in many programs, those responsible for accompanying the patient′s treatment either have not been recruited or lack proper training.[16] And finally it is felt that because of poor adherence of patients to self-administration of treatment, supervised therapy is the only way to ensure regularity of treatment. In tuberculosis, where poor adherence to therapy is a common phenomenon, directly observed treatment (DOTS) under supervision has been widely used as the ′standard cure′ in control programmes.[17]
DRUG REGIMENS IN LEPROSY
Currently two drug regimens have been officially recommended by the WHO:[3],[11]
1. WHO MDT-PB schedule, containing dapsone and rifampicin, and
2. WHO MDT-MB schedule, containing dapsone, clofazimine and rifampicin.
Although these regimens are effective, the need for new regimens that are more effective and operationally less demanding is being felt for many reasons.[18] From the operational point of view, the duration of MDT-MB is too long. Two of the components of MDT-MB, dapsone and clofazimine, are only weakly bactericidal, and clofazimine has some unpleasant side effects. Besides, as there is no clear indication that leprosy is a disappearing disease, the efforts to control leprosy must be sustained and adapted to the present situation.
It has been observed that research in the field of leprosy is shrinking.[19] The International Federation of Anti-leprosy Associations estimates that money spent on research has come down from US $6.5 million in 1990 to US $3 million in 1998. There is no industry interest in developing new drugs for leprosy.
NEWER DRUGS
Moxifloxacin (BAY 12-8039) is a new broad-spectrum fluoroquinolone that has been found to be the most active fluoroquinolone against M. tuberculosis in mice.[20] Studies have shown that moxifloxacin was far more bactericidal than ofloxacin against M leprae in mouse footpads.[21] The bactericidal activity of moxifloxacin was identical to that of a single dose of rifampicin.
Rifapentine (DL 473), a rifamycin derivative, is another drug which has pharmacokinetic properties far more favorable than rifampicin, with significantly higher peak serum concentrations and a much longer serum half-life.[22] In mouse footpads, rifapentine was observed to be more effective than a single dose of rifampicin or the combination of ROM in killing M. leprae.[23]
HMR 3647 (RU 66647, telithromycin) is a ketolide, a new class of macrolides possessing a 14 membered ring. It exhibits strong activity against gram-positive bacteria,[24] including mycobacteria, and displays significant bactericidal activity against M. leprae equal to or slightly greater than clarithromycin.[23]
A single dose combination of rifapentine, moxifloxacin and minocycline killed 99.9% of the viable M. leprae and was more bactericidal than a single dose of ROM or rifampicin alone.[23] In the same study, it was also observed that the combination of moxifloxacin-minocycline was more bactericidal than the combination of ofloxacin-minocycline.
Both moxifloxacin and rifapentine are being marketed in Europe indicating that their phase III studies have been performed. No data, however, is yet available regarding their efficacy in treating patients with leprosy.
The rifamycin analogues rifabutin and KRM-1648 were found to have significant anti-leprotic activity in mouse footpads.[25] Both the drugs were found to be more effective than rifampicin.
Monthly administered ROM for MB and PB leprosy:
Efficacy of once a month ROM in both MB (for 12 months) and PB (for 6 months) leprosy patient is being currently conducted in Myanmar, Guinea and Senegal. The final results will be available in mid 2007.[26]
TREATMENT OF LEPRA REACTIONS
Type 1 lepra reactionsThe mainstay of the treatment of type 1 lepra reaction continues to be corticosteroids. Although patients with upgrading type I lepra or reversal reactions (RR) are started on prednisolone 30-40 mg daily in a single dose, it was observed that 15-20 mg was the critical dose of prednisolone to control a RR after the initial high dose of corticosteroids.[27] In the field, as individual schedules are not feasible, semi-standardized schedules are preferred.[28] In a comprehensive review of data on reversal reactions, it was concluded that type 1 lepra reaction should be treated with prednisolone for periods longer than 12 weeks, probably up to 6 months. Long term-low dose steroid therapy was observed to be more useful than high dose-short term steroid therapy in the prevention of nerve damage.[29]
Type 2 lepra reactionsThe primary drug therapy of type 2 lepra reactions is with analgesics and corticosteroids. The WHO recommends the use of a standard course of prednisolone in a daily dose not exceeding 1 mg/kg body weight for a total duration of 12 weeks.[9] In patients who do not respond to corticosteroid therapy, clofazimine up to 300 mg/day may be added. This WHO report recommends the use of pentoxifylline alone or in combination with clofazimine and/or prednisolone.
However, the WHO categorically states that it does not support the use of thalidomide in type 2 lepra reactions, giving the following reasons.[30] Even at present, many thalidomide babies continue to be born. There is evidence that second generation babies with similar deformities are being born to thalidomide victims. Moreover, in patients of leprosy who were given thalidomide, reactions relapsed after discontinuation of the drug, and it is a non-essential drug in the treatment of type 2 lepra reactions. However, many workers differ from the WHO′s views, pointing out that the seventh report of the WHO expert committee on leprosy recommended thalidomide′s use for type 2 lepra reactions and that it is being used successfully in Brazil from 1965 onwards for such patients under supervision.[31] They also contend that thalidomide controls neuritis, relieves pain and improves nerve function, and it works faster than corticosteroids in ENL reactions.[32]
THALIDOMIDE ANALOGUES
Thalidomide′s adverse effects, such as teratogenicity, peripheral neuropathy, and drowsiness, among others, may limit its use. Hence, thalidomide analogues, which are chemically similar to thalidomide but appear to lack its side effects, are being pursued. Celgene Corporation of USA has developed two drugs, Revimid and Actimid, which have immunomodulatory properties. These drugs appear to be effective and promising as anti-myeloma agents. Although their use in leprosy is yet to be established, they may be useful for leprosy reactions.[33]
PREVENTION OF DISABILITIES
Leprosy results in a wide range of impairments, the most important ones being the result of damage to the peripheral nerves [Table - 1]. Peripheral nerve damage causes loss of sensory, motor and autonomic nerve function to the affected region, leading in turn to the primary deformity and, more importantly, secondary deformity, which results from repeated trauma and dryness and cracking of the skin. Although MDT has had a dramatic impact on the global prevalence of leprosy, there are still two to three million people with deformities worldwide.[3] In addition, in many parts of the world MDT′s impact on the rates of detection of new cases is unclear. Detecting, managing and understanding the mechanisms involved in nerve damage remain a high priority. In the early 1990s guidelines on the development, implementation and monitoring of Prevention of Disabilities (POD) were published. Leprosy programs that fail to prevent leprosy related disabilities are failing their patients and communities.[34]
The best ways to prevent disabilities, which are the result of the nerve damage, are early diagnosis and prompt treatment of leprosy reactions. Trials in prevention of disability (TRIPOD) are multi-centre, double blind, randomized, controlled trials to investigate the prevention and treatment of nerve damage in leprosy by using corticosteroid therapy. The early findings of these studies indicate that nerve function frequently recovers spontaneously and that prednisolone is safe, but there are limits to its usefulness.[35]
The ILEP has come out with a revised disability grading, with a special emphasis on eye involvement.[36] Most WHO/NLEP documents mention the simple ways to care for insensitive hands and feet, and the need for eye care and protection. All leprosy programs stress daily self-care of the effects of nerve function impairment, which is considered the responsibility of the individual patient. The role of the health care worker is to educate and enable patients in the self care process.[37]
INTEGRATION OF LEPROSY INTO GENERAL HEALTH SERVICES
It has been increasingly argued that with the declining prevalence of leprosy and shortened treatment regimens, general health care services could and should be able to manage leprosy without a significant increase in their workload.[38] Many agencies, including the World Bank and the WHO have argued that integrated intervention programs are more efficient and can be at least as effective as vertical programs and should therefore be the norm.[39]
Integration is considered more cost effective and feasible within national resources, thereby ensuring sustainability of leprosy services. In India, the integration of leprosy into general health services was seriously deliberated in the last decade. In July 1997, Tamil Nadu became the first state in India where the vertical NLEP was integrated with the primary health care/centre (PHC) system.[40] By 2001, more states were asked to rapidly integrate their programs in to the primary health care structure. Although experiences are diverse, several countries have shown that such integration is feasible and effective. In Sri Lanka, where such integration was completed in 2001, strong links were forged between central leprosy clinics, regional health authorities and dermatologists. Complications that could not be managed by health facility are being referred to the closest dermatological clinic.
This integration has already begun in India and will make dermatologists the only qualified leprosy specialists, as the vertical program of leprosy, including its medical specialists, will then be dismantled. Hence, it is imperative that dermatologists keep abreast of developments in the field of leprosy.
| 1. |
Chemotherapy of leprosy for control programs- report of WHO study. WHO technical report series No. 675;1982.
[Google Scholar]
|
| 2. |
Global situation of leprosy control at the beginning of the 21st century. Report of the international leprosy association technical forum. Lepr Rev 2002;73:S13-6.
[Google Scholar]
|
| 3. |
Final push strategy to elimination of leprosy as public health problem- questions and answers. WHO document, Second edition, Geneva, World health organization, 2003.
[Google Scholar]
|
| 4. |
Dhillon GP. Leprosy elimination in India. Bull lepr elimin alliance 2004;4:7-8.
[Google Scholar]
|
| 5. |
New leprosy case increase. News and notes. Lepr Rev 2003;74:92-7.
[Google Scholar]
|
| 6. |
WHO expert committee on leprosy, WHO technical report series, No. 874, 1998.
[Google Scholar]
|
| 7. |
Diagnosis and classification of leprosy. Report of the international association technical forum, Lepr Rev 2002;73:S17-26.
[Google Scholar]
|
| 8. |
Efficacy of single dose multi drug therapy for treatment of single lesion pauci bacillary leprosy. Single lesion multi-centred trial group. Indian J Lepr 1997;69:121-9.
[Google Scholar]
|
| 9. |
Modified guidelines on MDT regimen to be followed under NLEP. Directorate general (Lep), Nirman Bhavan, New Delhi 1997.
[Google Scholar]
|
| 10. |
Draft resolution, Sixth meeting of the WHO technical advisory group on elimination of leprosy (TAG), WHO Geneva, February 2004.
[Google Scholar]
|
| 11. |
Report on fifth meeting of the WHO technical advisory group on elimination of leprosy, World Health organization, Geneva, 2003.
[Google Scholar]
|
| 12. |
WHO expert committee on leprosy, WHO technical report series, No. 768, World Health Organization 1988.
[Google Scholar]
|
| 13. |
Operational guidelines on case detection, treatment, follow-up and reporting forms-NLEP, Leprosy division, Directorate general of health services, New Delhi 1992.
[Google Scholar]
|
| 14. |
Report on the third meeting of the WHO technical advisory group on elimination of leprosy. Brasilia, 1-2 February 2002, World health organization 2002.
[Google Scholar]
|
| 15. |
Ji B, Saunderson P. Uniform MDT regimen for all leprosy patients- another example of wishful thinking. Lepr Rev 2003;74:2-6.
[Google Scholar]
|
| 16. |
Chemotherapy. Report of the international leprosy association technical forum. Lepr Rev 2002;73:S27-34.
[Google Scholar]
|
| 17. |
Ji B. Accompanied MDT (A-MDT) - more questions than answers. Lepr Rev 2002;73:301-7.
[Google Scholar]
|
| 18. |
Grosset J. The new challenges for chemotherapy research. Workshop proceedings of leprosy research at new millennium. Lepr Rev 2000;71:S100-4.
[Google Scholar]
|
| 19. |
Strategic direction for research, TDR strategic direction: Leprosy, February 2002, World Health Organization, Geneva. WWW. TDR-leprosy. Web site.
[Google Scholar]
|
| 20. |
Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and vivo activities of Moxafloxacin and clonafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother 1998;42:2066-9.
[Google Scholar]
|
| 21. |
Grosset J. The new challenges for chemotherapy research. Work shop proceedings of leprosy research at new millennium. Lepr Rev 2000;71:S100-4.
[Google Scholar]
|
| 22. |
Ji B, Truffot-Pernot C, Lacriox C, Raviglione MC, O'Brien RJ, Olliaro P, et al. Effective ness of rifampin, rifabutin and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respi Dis 1993;148:1541-6.
[Google Scholar]
|
| 23. |
Hamilton-Miller JM, Shan S. Comparative invitro activity of ketolide HMR 3647 and four macrolides against gram-positive cocci of known erythromycin susceptibility status. J Antimicrob Chemother 1998;41:649-53.
[Google Scholar]
|
| 24. |
Dhople AM. Search for newer antileprosy drugs. Indian J Lepr 2000;71:7-22.
[Google Scholar]
|
| 25. |
Daumerie D. Current world health organization sponsored studies in the chemotherapy of leprosy. Workshop proceedings of leprosy research at new millennium. Lepr Rev 2000;71:S88-90.
[Google Scholar]
|
| 26. |
Naafs B, Pearson JM, Wheate HW. Reversal reactions: The prevention of permanent nerve damage. Comparison of short and long term steroid treatment. Int J Lepr 1979;47;7-12.
[Google Scholar]
|
| 27. |
Kiran KU, Stanley JN, Pearson JM. The out patient treatment of nerve damage in patients with borderline leprosy using a semi-standardized steroid treatment regimen. Lepr Rev 1985;56:127-34.
[Google Scholar]
|
| 28. |
Naafs B. Treatment duration of reversal reaction, a reappraisal. Back to the past. Lepr Rev 2003;74:328-36.
[Google Scholar]
|
| 29. |
Pannikar V. The return of thalidomide: new uses and renewed concerns. Lepr Rev 2003;74:286-8.
[Google Scholar]
|
| 30. |
Pereira GF. On thalidomide and WHO policies. In Commentaries. Lepr Rev 2003;74:288-90.
[Google Scholar]
|
| 31. |
Lockwood D, Bryceson A. The return of thalidomide: New uses and renewed concerns- reply. Lepr Rev 2003;74:290-4.
[Google Scholar]
|
| 32. |
Kaplan G. Potential of thalidomide and thalidomide analogues as immuno modulatory drugs in leprosy and leprosy reactions. Lepr Rev 2000;71:S117-20.
[Google Scholar]
|
| 33. |
Smith CM, Smith WC. Current understanding of disability prevention. Indian J Lepr 2000;73:393-9.
[Google Scholar]
|
| 34. |
Editorial. Lepr Rev 2003;74:297-9.
[Google Scholar]
|
| 35. |
Brandsma JW, Van Brakel WH. WHO disability grading: Operational definitions. Lepr Rev 2003;74:366-73.
[Google Scholar]
|
| 36. |
Prevention of disabilities and rehabilitation. Report of the International leprosy association technical forum. Lepr Rev 2002;73:S35-43.
[Google Scholar]
|
| 37. |
Editorial. Integration of leprosy services, Lepr Rev 2002;73:109-10.
[Google Scholar]
|
| 38. |
Saunderson PR, Ross WF. Training for integration. Lepr Rev 2002;73:130-7.
[Google Scholar]
|
| 39. |
Rao PS, Gift N, Rao GS, Samuel P, Bushanam RS. Elimination of leprosy: Integration of leprosy related activities into general health services of Tamil Nadu. Lepr Rev 2002;73:123-9.
[Google Scholar]
|
| 40. |
Kasturiaratchi ND, Settinayke S, Grewal P. Process and challenges: How the Sri Lankan health system managed the integration of leprosy services. Lepr Rev 2002;73:177-85.
[Google Scholar]
|
Fulltext Views
6,732
PDF downloads
3,511





