Translate this page into:
Refractory subacute cutaneous lupus erythematous responding to a single course of belimumab: A new anti-BLyS human monoclonal antibody
Correspondence Address:
Husein Husein-ElAhmed
Department of Dermatology, San Cecilio University Hospital, Granada
Spain
| How to cite this article: Husein-ElAhmed H, Callejas-Rubio JL, Rios-Fernandez R, Ortego-Centeno N. Refractory subacute cutaneous lupus erythematous responding to a single course of belimumab: A new anti-BLyS human monoclonal antibody . Indian J Dermatol Venereol Leprol 2014;80:477-478 |
Sir,
Subcutaneous lupus erythematosus (SCLE) is a chronic autoimmune inflammatory disease which includes a variety of specific skin lesions involving the upper portion of back and chest that, in certain cases, may be disfiguring. The relapsing and remitting pattern of this condition along with the clinical heterogeneity makes SCLE a challenge to manage. Despite clear advances in the understanding of this condition, certain patients are refractory to conventional drugs. Belimumab is a human monoclonal antibody approved by the FDA in March 2011 for SLE with active disease despite standard treatments. [1] This agent is the first in a new class of drugs known as B-lymphocyte stimulator (BLyS)-specific inhibitors. We report a case of treatment-resistant SCLE showing a satisfactory long-term response to belimumab infusion.
A 52-year-old woman presented with a 3 year history of pruritic, scaly eruption over her upper back and chest. Six years ago, she was diagnosed with SLE with kidney and musculo-skeletal involvement. Clinical examination revealed severely erythematous infiltrated scaly plaques and patches in a predominantly photosensitive distribution on the upper third of the chest [Figure - 1]. A skin biopsy revealed vacuolar alteration of the basal cell layer and a lymphocytic cell infiltrate around vessels confirming the diagnosis of SCLE. Laboratory tests showed an erythrocyte sedimentation rate (ESR) of 100 mm/h, C-reactive protein of <0.3 mg/dL (normal), hemoglobin of 11.1 g/dL, white blood count of 4900/mm 3 , serum urea of 37 mg/dL and serum creatinine of 0.8 mg/dL. She was positive for antinuclear (1/640) and for anti-Ro/SSA antibodies. Previous unsuccessful therapies for skin disease included topical clobetasol, tacrolimus, pimecrolimus, antimalarials, methotrexate and azathioprine. At this point, mycophenolate mofetil 2 g daily was initiated. After 10 weeks, no response was observed and the drug was discontinued due to gastrointestinal adverse effects. She was then administered cyclophosphamide pulse therapy (500 mg/m 2 ), but it was discontinued after 7 weeks for lack of improvement. Thalidomide was not considered due its potential severe side effects. Use of intravenous immunoglobulins had a remarkably rapid effect on skin lesions but clinical benefit was not persistent and recurrence was observed six weeks after infusion. Despite these medications, her skin lesions and arthritis worsened. In addition, myalgia and fatigue appeared. She received rituximab infusion at a dose of 1 gm at days 1, 15, 30 and 45. The rash and complaints improved significantly. Maintenance therapy with hydroxychloroquine 200 mg daily and prednisone 20 mg daily was initiated. Three months later, a recurrence of skin lesions in a similar distribution was observed. At this point we decided to start belimumab therapy at a dose of 10 mg/kg/day on days 1, 14 and 28. Dramatic clinical improvement was achieved after the second dose of belimumab. The treatment was well tolerated with no significant side effects. By the third infusion of belimumab, lesions cleared completely without any post-inflammatory pigmentation [Figure - 2]. No further lesions or recurrence have been observed after one year of follow-up.
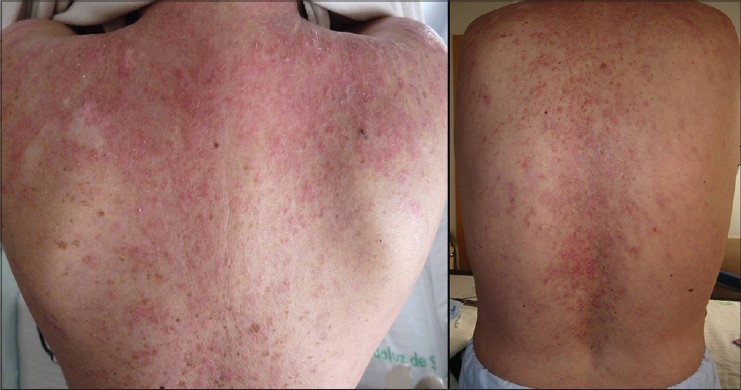 |
| Figure 1: Scaly erythematosus papules and patches involving the upper third of back and shoulders |
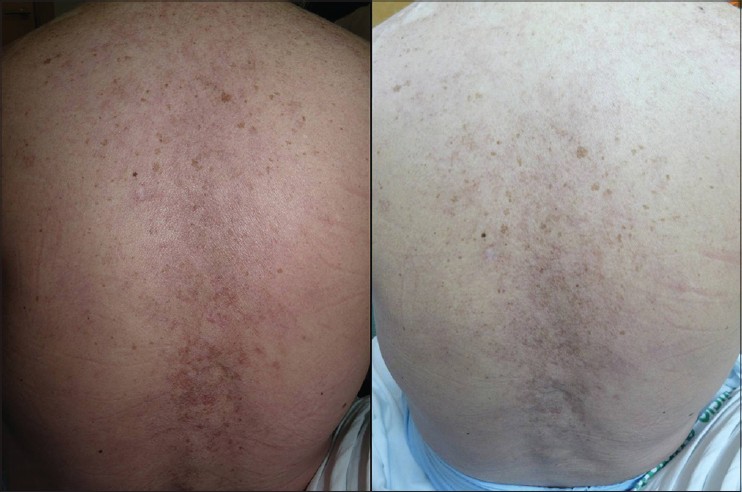 |
| Figure 2: Dramatic clearance of lesions after the third infusion of belimumab |
SCLE is one of the LE-specific skin lesions which consists of non-scarring papulosquamous or annular skin lesions in a characteristic photo-distribution. Three quarters of SCLE can be managed with conventional topical or/and systemic therapies while in the remaining quarter of cases, skin lesions can be particularly severe and refractory to treatment. [2] BLyS is a major regulatory factor which controls the development and survival of B lymphocytes. Current evidence has identified that BLyS family of ligands and receptors play a central role in the B-cell pathogenesis of SLE. [3] Belimumab is a fully human monoclonal antibody that inhibits the binding of soluble circulating BLyS to its target receptors (BR3, TACI, BCMA) on B cells. The ultimate goal is to block the crucial survival signal in the early stages of B lymphocyte development decreasing the survival of auto-reactive B lymphocytes. [3] An overexpression of BLyS in patients with SLE has been observed with a potential correlation between serum levels of BLyS and SLE disease activity. [4] In our patient, the use of conventional topical and systemic therapies did not lead to clinical improvement. Although intravenous immunoglobulins showed an initial good response, we observed a rapid relapse of lesions. Rituximab is an anti-CD 20 monoclonal antibody which results in major clinical and biochemical improvement for patients with resistant SLE and SCLE. In our patient, a remission was achieved with rituximab although a relapse was observed in spite of standard maintenance therapy. We decided to start a rescue treatment based on a new class of drugs which have a different mechanism of action compared to previous approved therapies. Belimumab is a specific inhibitor of BLyS and this novel way to manage the autoimmune environment gives physicians a valuable alternative for refractory cases. Our patient achieved a stable and long-term remission only when belimumab therapy was initiated. The highly specific mode of action of belimumab may be responsible for these significant clinical outcomes. The standard protocol for belimumab administration is as an intravenous infusion over 1 hour on days 1, 14 and 28, and then every 28 days. [5] In our patient, the first three infusions at 2-week intervals led to an excellent clinical response with no need to initiate the doses at 4-week intervals. Similar to other immunomodulatory therapies, belimumab may increase the incidence of infection. No such side effects were noted in our patient although care should be exercised when administering belimumab to patients with chronic infections. If a new infection develops during belimumab therapy, temporary cessation of treatment is recommended. [5] Belimumab may be a promising new approach in the management of therapy-resistant SCLE.
| 1. |
Human Genome Sciences, Inc. Center for drug evaluation and research. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125370Orig1s000Lbl.pdf. [Last accessed on 2011 Mar 29].
[Google Scholar]
|
| 2. |
Zampieri S, Alaibac M, Iaccarino L, Rondinone R, Ghirardello A, Sarzi-Puttini P, et al. Tumour necrosis factor alpha is expressed in refractory skin lesions from patients with subacute cutaneous lupus erythematosus. Ann Rheum Dis 2006;65:545-8.
[Google Scholar]
|
| 3. |
Kim SS, Kirou KA, Erkan D. Belimumab in systemic lupus erythematosus: An update for clinicians. Ther Adv Chronic Dis 2012;3:11-23.
[Google Scholar]
|
| 4. |
Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 2008;58:2453-9.
[Google Scholar]
|
| 5. |
Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721-31.
[Google Scholar]
|
Fulltext Views
4,737
PDF downloads
2,170





