Translate this page into:
Response to ixekizumab in a patient of psoriasis with secondary failure to secukinumab
Corresponding author: Dr. Saurabh Mittal, Department of Dermatology, NMC Royal Hospital, Abu Dhabi, UAE. drsaurabh0811@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mittal S. Response to ixekizumab in a patient of psoriasis with secondary failure to secukinumab. Indian J Dermatol Venereol Leprol 2021;87:119-21.
Sir,
Psoriasis is a chronic inflammatory disorder of the skin with multifactorial etiopathogenesis and an unknown etiology. Recent research in understanding the nature of the disease has highlighted the importance of TNF-a, IL-23 and IL-17 in the pathogenesis. This has helped in the development of multiple targeted biological therapies that influence the levels of these cytokines resulting in excellent therapeutic responses. The uniqueness of this case lies in the fact that different biological agents targeting the same cytokine, can be used in patients if one molecule fails.
A 34-year-old Filipina woman presented, in May 2018, as a known case of severe psoriasis (Psoriasis Area and Severity Index (PASI) = 12.4 and body surface area = 20%) for last 12 years. She also reported occasional back pain in the past. She had received multiple treatments such as phototherapy, methotrexate and acitretin. She was finally introduced to biologicals, intially with ustekinumab and later secukinumab. The patient was started on secukinumab in 2017 along with methotrexate 10 mg weekly, which she had been on intermittently for the last 9 years). The patient reported excellent clinical response with resolution of back pain and no side effects.
At the time of presentation, her PASI was 12.4 (due to gaps in treatment) with thick, erythematous plaques on the lower legs that had been persistent ever since onset. She was restarted on a maintenance dose of secukinumab 300 mg per month, with gradual tapering down of her methotrexate dosage and eventually stopping it over the next 2 months. The patient responded to the therapy with only the nail changes and lesions on the legs persisting.
The patient was stable for almost 8 months with her PASI reducing to 6.0 and her body surface area involvement reducing to 8%. However, in the 9th month after initiation of secukinumab, the patient presented with a sudden flare of her disease and her PASI and body surface area increased to 21.3 and 35%, respectively [Figure 1]. There were no identifiable precipitating factors for such deterioration.
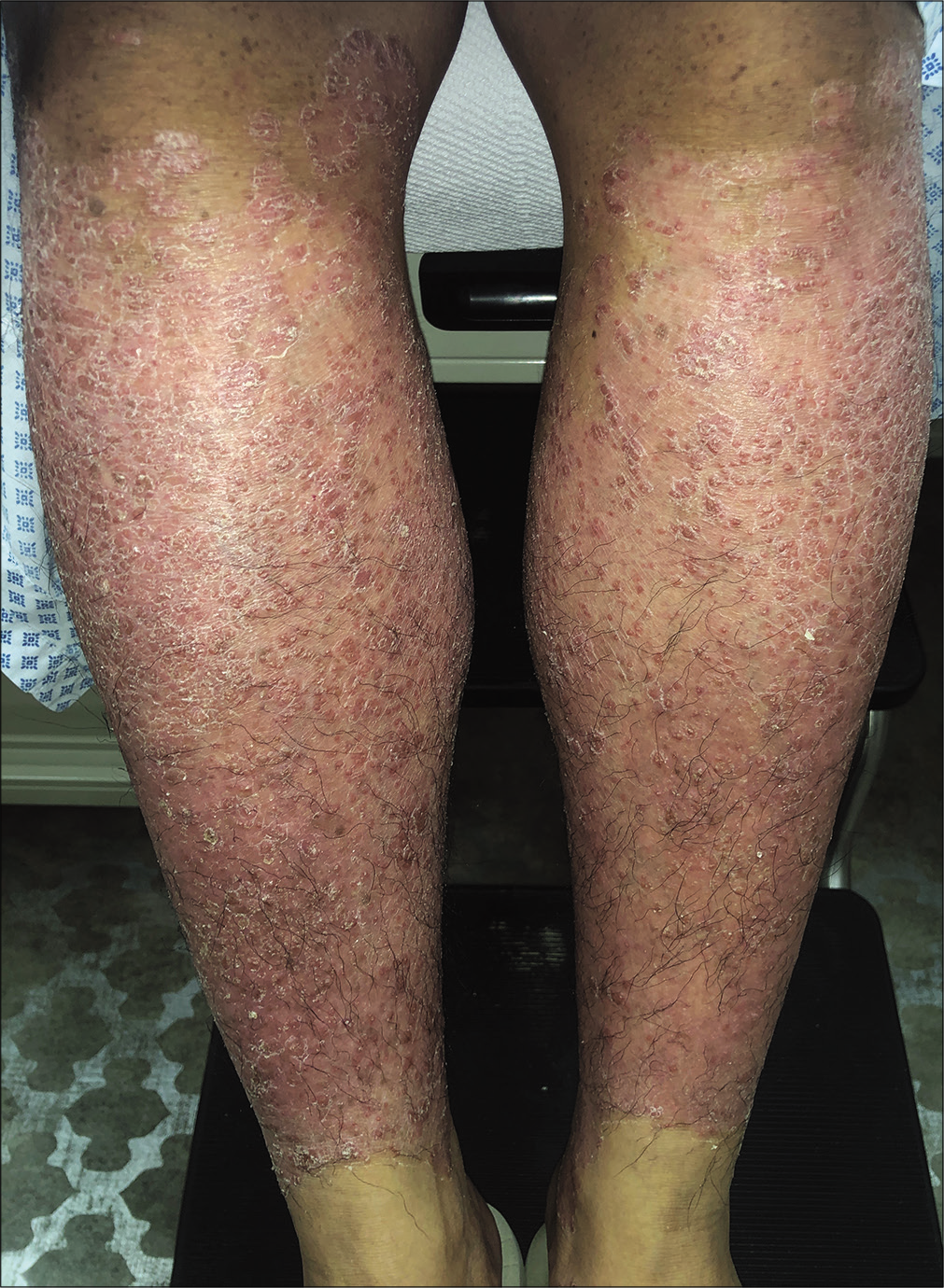
- Lesions before starting ixekizumab
The patient was then switched to ixekizumab. She returned 2 weeks after taking the 1st dose of 160 mg and reported significant improvement, including in the lesions on the legs. Her PASI reduced to 9.6 from 21.3 within 2 weeks [Figure 2].
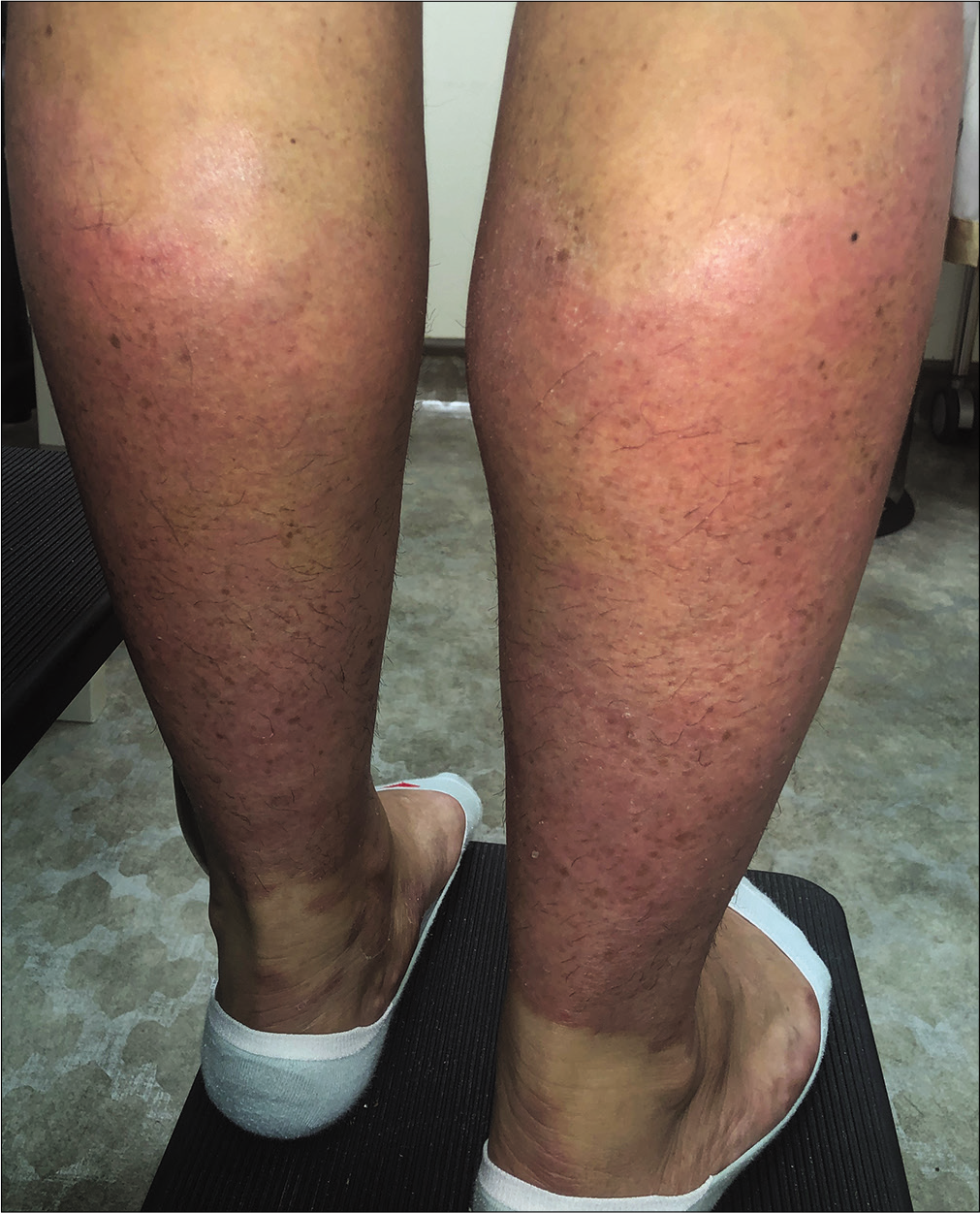
- Lesions 2 weeks after a single dose of ixekizumab
She was continued on a dose of 80 mg every fortnight for another 10 weeks and at the end of 12 weeks after starting ixekizumab, her PASI had reduced to 2.3 with a body surface area of 11%. This had been by far the best outcome of her disease as per the patient. She is now on an 80 mg 4 weekly maintenance dose. The patient has only reported mild injection site reactions to ixekizumab with no other adverse effects.
Psoriasis is a chronic systemic inflammatory disorder associated with multiple comorbidities including, but not limited to, obesity, metabolic syndrome and psychiatric manifestations.1 TNF-a, IL-23 and IL-17 play a central role in the pathogenesis of the condition and their levels are dysregulated in psoriatic patients. This pathway upregulates the levels of IL-17 through the activation of Th17 cells.2 The levels of IL-17 are elevated in both the serum and skin lesions of patients with active disease.3
IL-17A inhibitors, such as secukinumab and ixekizumab, have consistently been proven in different studies to be superior to placebo and etanercept in reducing the PASI and improving the quality of life, while being a safe modality of treatment. Notably, the most common adverse events have been nasopharyngitis, headache, upper respiratory tract infection and diarrhea. Mild and transient neutropenia without associated infections has also been noted. Serious adverse effects observed were stroke and myocardial infarction.3
Secukinumab is a fully human monoclonal antibody that binds and neutralizes IL-17A. Failure of therapy with secukinumab has been reported and many such patients have been shifted to ixekizumab with good response, like in our case.4 Ixekizumab, like secukinumab, targets IL-17A with a difference that it is humanized rather than being fully human. This difference may account for its higher immunogenicity. Besides, ixekizumab shows higher affinity and stability to IL-17A than secukinumab.4 It has also been proposed that the two antagonists may be targeting different epitopes of IL-17, thus leading to variation in response.5 This might explain the response to ixekizumab in our patient who failed secondarily on secukinumab and for the faster onset of action with the former.
Acknowledgment
A patient who is the inspiration source for this manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal the identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. 2017;76:377-90.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-17 alters the biology of many cell types involved in the genesis of psoriasis, systemic inflammation and associated comorbidities. Exp Dermatol. 2018;27:115-23.
- [CrossRef] [PubMed] [Google Scholar]
- Secukinumab in the treatment of psoriasis and psoriatic arthritis: A review of the literature. Skin Therapy Lett. 2017;22:1-6.
- [Google Scholar]
- Clinical experience of ixekizumab in the treatment of patients with history of chronic erythrodermic psoriasis who failed secukinumab: A case series. Br J Dermatol. 2019;181:1106-7.
- [CrossRef] [PubMed] [Google Scholar]
- IL-17A inhibitor switching-efficacy of ixekizumab following secukinumab failure. A single-center experience. Acta Derm Venereol. 2019;99:769-73.
- [CrossRef] [PubMed] [Google Scholar]





