Translate this page into:
Reticular telangiectatic erythema associated with implantable automatic cardioverter defibrillator
Correspondence Address:
Ximena Calderón-Castrat
Paseo San Vicente 58-182, 37007 Salamanca
Spain
| How to cite this article: Calderón-Castrat X, Cañueto J, Román-Curto C, Santos-Briz &, Fernández-López E. Reticular telangiectatic erythema associated with implantable automatic cardioverter defibrillator. Indian J Dermatol Venereol Leprol 2018;84:334-336 |
Sir,
Reticular telangiectatic erythema associated with the implantation of a medical device is a rare entity, which must be considered as one of the differential diagnoses of a local infection or allergic contact dermatitis to prevent future extractions and/or replacement of the devices.[1]
A 64-year-old woman with a medical history of idiopathic dilated cardiomyopathy and cardiac insufficiency developed an erythematous plaque on the chest, 1 week after the subcutaneous placement of an implantable automatic cardioverter defibrillator [BIOTRONIK LUMAX 340 HFT], overlying the site of implantation. Infection of the implanted device was suspected and the patient received wide spectrum antibiotic therapy with amoxicillin and clavulanate without much improvement. Subsequently, the patient underwent a second surgical procedure where the implantable automatic cardioverter defibrillator was relocated at the same site but in a submuscular placement; however, the lesion persisted.
Routine blood examination findings were within normal limits and both blood cultures and intra-operative cultures were negative. The patient was referred to the dermatologist after an interval of 1 month, with a possible diagnosis of refractory cellulitis. Physical examination revealed a slightly warm, non-indurated, erythematous plaque with ill-defined margins and a reticulate appearance (more evident with dermoscopy), located on the left side of the chest [Figure - 1]. The device could be felt on palpation, which had been previously deeply implanted and there was no loco-regional lymph node enlargement. No systemic or local symptoms were observed. In view of the absence of clear signs of infection, a skin biopsy was performed. The histopathological study revealed spongiotic dermatitis with telangiectatic vessels in the papillary dermis and a mixed inflammatory infiltrate. These findings suggested reticular telangiectatic erythema [Figure - 2]. The concomitant antibiotics were discontinued and therapy was initiated with methylprednisolone aceponate cream once a day. The erythematous plaque showed clinical improvement progressively and after 1-week, complete resolution was observed [Figure - 3]. Corticosteroid treatment was suspended after 2 weeks and no immediate recurrence was observed. A 6-month follow-up revealed complete remission.
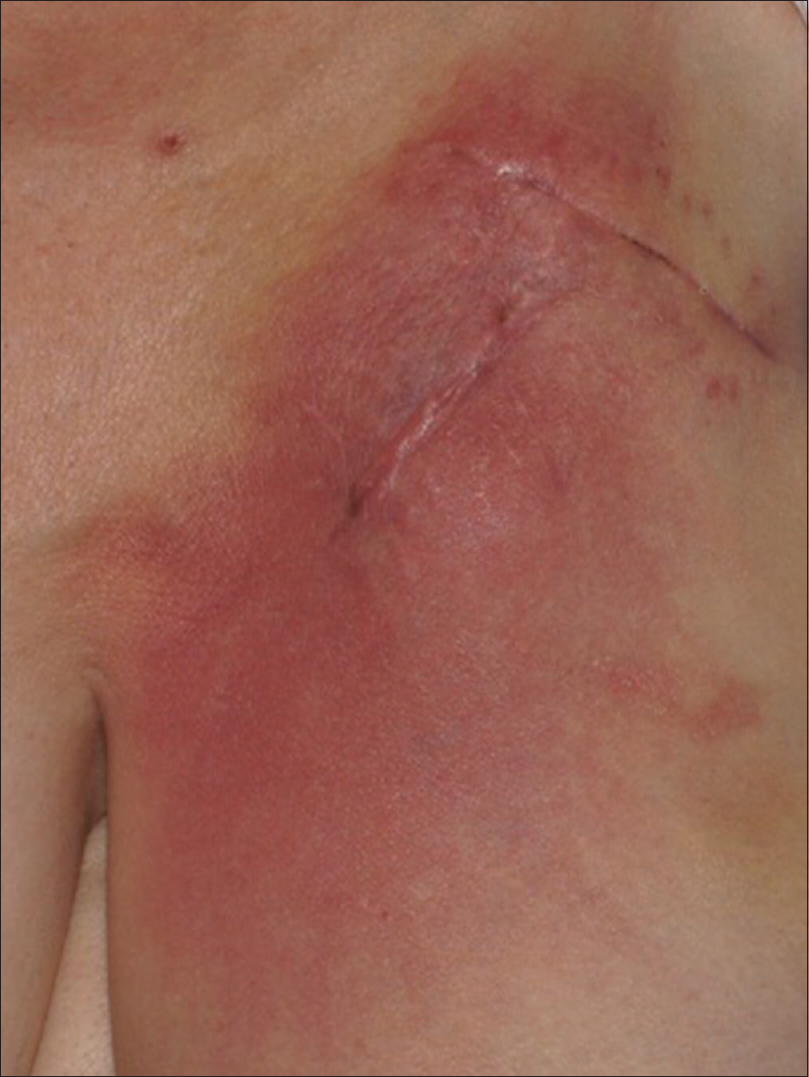 |
| Figure 1: Reticular telangiectatic erythema: Erythematous plaque located on the left side of the chest, showing the scar at the insertion site and the superior border of the subcutaneous implantable cardioverter defibrillator |
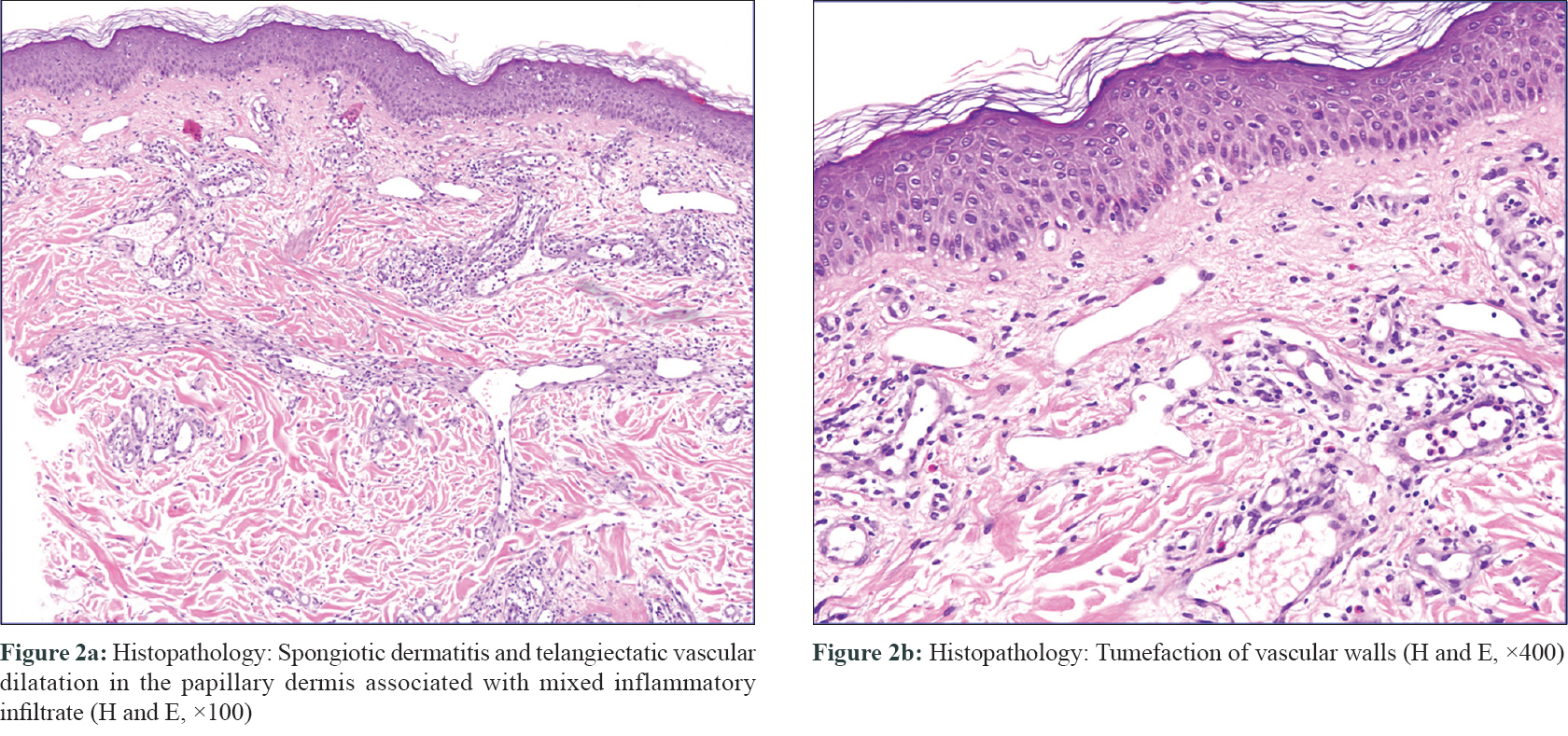 |
| Figure 2 |
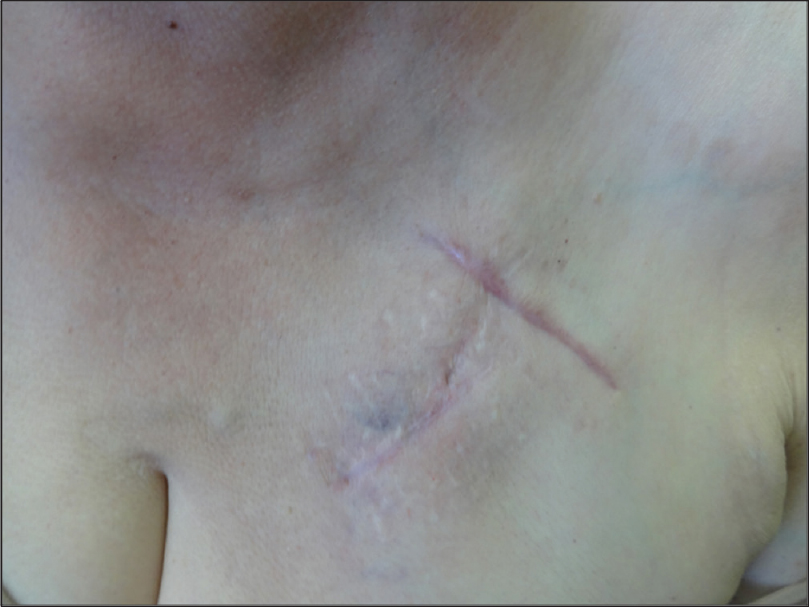 |
| Figure 3: Resolution of reticular telangiectatic erythema after treatment with topical corticosteroid cream |
In 1981, the first case of reticular telangiectatic erythema was described by Gensch and Schmitt.[1] This rare entity is characterized by painless erythema with slightly prominent telangiectasia, associated with the placement of a medical device.[1],[2] Most of the cases found in medical literature appear after placement of an implantable automatic cardioverter defibrillator or a pacemaker, however, some cases have also been described with prostheses and infusion pumps.[2] Reticular telangiectatic erythema may develop weeks or even months after the intervention.[2],[3],[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16] Although its pathophysiology is not completely clear, some authors suggest that it is likely due to the development of changes in the microcirculation secondary to the healing process, generating an obstruction of blood flow caused by the device or by the anatomical characteristics of the implanted sites.[2],[7],[15] Allergic contact dermatitis to some of the components of the devices is one of the entities which must be taken into account as a differential diagnosis, besides local infection.[3],[4],[5] According to the literature, however, it is not necessary to perform skin tests in all patients for confirmation of diagnosis.[6],[7],[8],[9] The clinical course of reticular telangiectatic erythema consists of spontaneous resolution,[9],[12],[14] with or without treatment, as observed in our patient; whereas in other cases, disappearance or partial improvement is seen after device removal.[2],[4],[6],[8],[11] In any case, therapeutic management should always include reassurance to the patient and observation.
There are only a few described cases of reticular telangiectatic erythema associated with medical devices.[2],[15] However, it is believed that this low incidence rates may be attributed to missed diagnosis due to lack of awareness.[7]
We performed a review of all the cases reported in medical literature after implantation of a cardiac device and found a total of 26 cases. Twenty (77%) cases of reticular telangiectatic erythema occurred in male patients and 2 (7,6%) in women (sex was not available in 4 patients). Time of onset for the development of reticular telangiectatic erythema was available for 17/26 cases; it was observed in 9 (53%) within the first 3 months after implantation and in 15 (88.2%) within the first 2 years. Only in 2 cases, reticular telangiectatic erythema was observed after 4 or 5 years.[3],[6] Histopathological assessment was performed in 21 (80.7%) patients, which was consistent with telangiectatic blood vessels in the papillary dermis and superficial perivascular lymphohistiocytic infiltrate. Patch testing was carried out in 18 (69.2%), showing negative results in all cases. Nine (35%) patients received treatment. In patients receiving topical corticosteroids and/or oral antibiotics, no changes were observed in the reticular telangiectatic erythema, except in our case. On the other hand, with replacement or removal of the device performed in 5 (19.2%) patients, 3 (60%) experienced resolution, 1 (20%) partial improvement, and 1 (20%) showed no changes.[2],[6],[10],[11]
Furthermore, the general course of reticular telangiectatic erythema without treatment in 12 (46.1%) patients revealed no changes in 6 (50%),[1],[7],[15],[16] spontaneous resolution in 3 (25%),[9],[12],[14] and partial improvement in 3 (25%).[2],[4],[8]
In summary, we present a new case of reticular telangiectatic erythema with a successful outcome. Dermatologists and specialists in medical device placement, such as cardiologists and traumatologists, should be familiar with this benign clinical entity as it may prevent aggressive procedures involving unnecessary replacement or extraction, as in our case.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gensch EG, Schmitt CG. Circumscribed reticular telangiectatic erythema following implantation of a heart pacemaker. Hautarzt 1981;32:651-4.
[Google Scholar]
|
| 2. |
Aneja S, Taylor JS, Billings SD, Honari G, Sood A. Post-implantation erythema in 3 patients and a review of reticular telangiectatic erythema. Contact Dermatitis 2011;64:280-8.
[Google Scholar]
|
| 3. |
Kopera D, Auer-Grumbach P, Cerroni L, Smolle J. Pacemaker erythema with telangiectasis. Hautarzt 1994;45:716-8.
[Google Scholar]
|
| 4. |
Krasagakis K, Vogt R, Tebbe B, Goerdt S. Persistent telangiectatic erythema associated with an automatic implantable cardioverter defibrillator. Br J Dermatol 1997;136:633.
[Google Scholar]
|
| 5. |
Wimmershoff MB, Landthaler M, Stolz W. The artificial pace- maker erythema. Dtsch Med Wochenschr 1998;123:441.
[Google Scholar]
|
| 6. |
Dinulos JG, Vath B, Beckmann C, Welch MP, Piepkorn M. Reticular telangiectatic erythema associated with an implantable cardioverter defibrillator. Arch Dermatol 2001;137:1259-61.
[Google Scholar]
|
| 7. |
Herbst RA, Weiss J. Reticular telangiectatic erythema associated with an implantable cardioverter defibrillator: an underpublished entity? Arch Dermatol 2003;139:100-1.
[Google Scholar]
|
| 8. |
Lin YC, Chiu HC, Chu CY, Sun CC. Telangiectatic pacemaker erythema. Clin Exp Dermatol 2003;28:447-8.
[Google Scholar]
|
| 9. |
Pitarch G, Mercader P, Torrijos A, Martínez-Menchón T, Fortea JM. Reticular telangiectatic erythema associated with an implantable cardioverter defibrillator. Cutis 2006;78:329-31.
[Google Scholar]
|
| 10. |
García SM, González IR, Sambucety PS, Rodríguez Prieto MA. Reticulated telangiectatic erythema associated with automatic implanted defibrillator. J Eur Acad Dermatol Venereol 2008;22:115-6.
[Google Scholar]
|
| 11. |
Martin LK, Wendschuh P, Wendschuh P. Reticulated telangiectatic erythema of the pacemaker. Pacing Clin Electrophysiol 2008;31:624-6.
[Google Scholar]
|
| 12. |
Rodríguez-Lojo R, Verea MM, Godoy J, Barja JM. Reticular telangiectatic erythema in a patient with a cardioverter defibrillator. Actas Dermosifiliogr 2010;101:183-4.
[Google Scholar]
|
| 13. |
Hinterberger L, Müller CS, Vogt T, Pföhler C. Reticulated teleangiectatic erythema after implantation of medical devices. An increasingly occurring phenomenon? Hautarzt 2011;62:770-3.
[Google Scholar]
|
| 14. |
Ringrose JS, Banerjee T, Hull PR. Angiosarcoma-like presentation of pacemaker-related vascular proliferation. Clin Exp Dermatol 2012;37:143-5.
[Google Scholar]
|
| 15. |
Ocampo OV, Marín VM, Idarraga JC. Reticulated telangiectatic erythema related to an implantable carioverter defibrillator: Case report and review of the literature. Dermatol Argent 2012;18:49-52.
[Google Scholar]
|
| 16. |
Beutler BD, Cohen PR. Reticular telangiectatic erythema: Case report and literature review. Dermatol Pract Concept 2015;5:71-5.
[Google Scholar]
|
Fulltext Views
3,402
PDF downloads
3,298





