Translate this page into:
Revisiting the role of the slit-skin smear in the diagnosis of Indian post-kala-azar dermal leishmaniasis
2 Parasitology Laboratory, National Institute of Pathology (Indian Council of Medical Research), New Delhi, India
Correspondence Address:
Aradhana Bhargava
Apex Regional STD Teaching, Training and Research Centre, Room No. 549, 5th Floor, New OPD Building, Safdarjung Hospital, New Delhi - 110 009
India
| How to cite this article: Bhargava A, Ramesh V, Verma S, Salotra P, Bala M. Revisiting the role of the slit-skin smear in the diagnosis of Indian post-kala-azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol 2018;84:690-695 |
Abstract
Background: Post kala azar dermal leishmaniasis (PKDL) is a neglected dermatosis that develops as a sequel to kala azar after apparent complete treatment. Being a non life threatening condition, patients often delay treatment thereby maintaining a reservoir of infection. The diagnosis of PKDL rests on the demonstration of the parasite in tissue smears, immune diagnosis by detection of parasite antigen or antibody in blood, or detection and quantitation of parasite DNA in tissue specimens. Sophisticated molecular tests are not only expensive but also need skilled hands and expensive equipment. To be useful, diagnostic methods must be accurate, simple and affordable for the population for which they are intended.
Aims: This study was designed to assess functionality and operational feasibility of slit-skin smear examination.
Methods: Sensitivity and specificity was evaluated by performing slit-skin smear and histo-pathological examination in 46 PKDL patients and the results were compared with the parasite load in both the slit aspirate and tissue biopsy specimens by performing quantitative Real-time PCR (Q-PCR).
Results: The slit-skin smear examination was more sensitive than tissue biopsy microscopy. The parasite loads significantly differed among various types of clinical lesions (P < 0.05). The threshold of parasite load for detection by SSS microscopy was 4 parasites/μl in slit aspirate and 60 parasites/μg tissue DNA in tissue biopsy while that for tissue microscopy was 63 parasites/μl and 502 parasites/μg tissue DNA respectively. As detection of Leishmania donovani bodies may be challenging in inexperienced hands, the microscopic structure of these has been detailed along with a comprehensive discussion of pre analytical, analytical and post analytical variables affecting its identification. To facilitate the diagnosis of PKDL, some scenarios have been suggested taking into consideration the clinical, epidemiological, immunological and microscopic aspects.
Conclusion: Such evidence based medicine helps minimize intuition, systematize clinical experience and provides a diagnostic rationale as sufficient grounds for a clinical decision.
Introduction
The National Health Policy set the goal for kala-azar elimination in India by the year 2010, which was later revised to 2015. Undiagnosed and untreated patients of PKDL are a reservoir of infection wherein the parasites are easily accessible to the vector, i.e. the sand fly, for transmission.[1] Thus, an important element in the elimination of kala-azar is early diagnosis and management of both cases and reservoir.
Laboratory diagnosis of PKDL can be performed by direct microscopy, immunological techniques, rapid tests for antibody detection, and molecular methods.[2] The choice of test, however, depends upon the available infrastructure, simplicity, and reliability of the method. The slit skin smear preparation is an old and reliable technique for the diagnosis of PKDL that can be performed by the clinician as an office procedure; it is still considered the gold standard test, along with culture.[2]
This article reiterates the role of the slit skin smear in the diagnosis of PKDL based on our experience and explains the performance and operational characteristics, functionality, methodology, and diagnostic criteria along with the difficulties encountered while performing this examination.
Slit-Skin Smear Examination
Performance characteristics
The basic performance characteristics of a test designed to distinguish the infected from the uninfected is the sensitivity. The sensitivity for the slit-skin smear is in the range of 67–100% in nodules, 36–69% for papules, and 7–33% in macules.[3],[4],[5] Nevertheless, recognizing Leishman-Donovan (LD) bodies in a well-made smear requires patience and experience. The organisms, when present, are often found only in indurated lesions and even under ideal conditions the success rate has varied from as low as 20% in Sudanese[6] to approximately 60% in Indian PKDL.[7] The polymorphic presentation, where the degree of inflammation and parasite burden is least in macules, increasing from papules to nodules may explain the smear negativity.[8]
Out of a 50 patients of PKDL, we performed slit-skin smear examination and histopathology in 46 patients as 4 paediatric patients refused to give biopsy sample. The parasite load was studied in both slit-skin aspirate and tissue biopsy specimens by performing SYBR Green I - based Leishmania specific quantitative real-time polymerase chain reaction (QPCR), as described by Verma et al.[9] The real-time PCR assay accurately quantifies the parasite load and has a detection limit of 1 fg parasite DNA per reaction (corresponding to 0.01 parasite). It was reported as the number of Leishmania parasites/μg tissue DNA in the case of biopsy specimens and as the number of parasites/μl slit aspirate in the aspirates. The slit-skin aspirate and biopsy specimens were also microscopically examined for the presence of LD bodies.
As seen in [Table - 1], nodular form was the most frequently encountered presentation. 80% patients with past history of kala-azar of duration 0-5 years presented with papular forms of lesions.
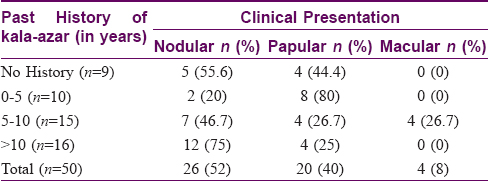
All macular lesions (n = 4) were negative on histopathology and slit-skin smear examinations. Only 2/17 (11.7%) papular lesions were positive on histopathology as compared to 10/20 (50%) slit-skin smear specimens. In nodular lesions, 13/26 (50%) histopathology specimens and 20/26 (76.9%) slit-skin smear specimens were positive. These results clearly demonstrate the higher sensitivity of the slit-skin smear for screening PKDL cases.
An analysis of the results of the quantitative PCR based on the type of lesion, showed a significant (P < 0.0001) positive correlation (Pearson correlation coefficient = 0.82) between the parasite load of the tissue biopsy and slit aspirate from the same PKDL cases. In slit aspirates the parasite load of macular versus papular (P = 0.009), macular versus nodular (P = 0.003), papular versus nodular (P = 0.004) lesions were significantly different. Similarly, there was a significant difference in tissue biopsy parasite load between macular versus nodular (P = 0.009), and papular versus nodular (P < 0.0001) lesions. However, the tissue biopsy parasite load between macular and papular (P = 0.206) lesions did not differ significantly.
The slit skin aspirate parasite load and tissue biopsy parasite load of histopathology positive and negative cases differed significantly (P = 0.0008 and 0.03 respectively) while the findings of slit skin smear were insignificant of the parasite load [Table - 2].
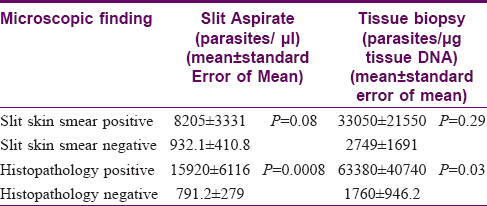
A slit skin smear preparation involves taking a scraping of the dermis through a small cut over the skin lesion where the parasite resides. Despite the varying load of parasites, this undiluted and unprocessed sample is well suited to reveal amastigote forms on microscopic examination. The significant difference in the parasite load of the histopathology negative and positive cases may be due to alterations in parasite density during storage, processing, and staining. During processing the tissue passes through several dehydrating solutions causing the inflammatory cellular infiltrates to appear compact.[10] Moreover, histopathology samples include the epidermis and the full thickness of the dermis as a result of which the material gets diluted and contains fewer Leishmania organisms.[10] It has been reported that Giemsa stain examination of slit-skin or touch smears enables better evaluation and recognition of organisms in direct microscopy than formalin-fixed paraffin embedded histopathology sections in PKDL[7] as well as cutaneous leishmaniasis[10],[11].
Considering the lowest parasite load detected by microscopy as the threshold limit, the positivity for slit skin smear was 4 parasites/μl slit aspirate and 60 parasites/μg DNA in tissue biopsy samples. However, the detection limit for tissue biopsy microscopy was 63 parasites/μl slit aspirate and 502 parasites/μg DNA in tissue biopsy samples. [Figure - 1] shows that slit skin smear examination is more sensitive compared to tissue biopsy microscopy as its threshold for positivity is much less. During slit skin smear preparation, material for direct microscopy was first smeared on at least 2 slides and the blade was finally dropped into a vial for estimating parasite load after scraping the same site. This may have influenced the results leading to direct microscopy being positive in those showing lower parasite loads. The biopsy sample was taken from a similar but different lesion. The study highlights the fact that although almost all LD body positive histopathology were also positive on slit skin smear, the reverse was not true: of the 30 smear positive cases only 14 were positive on histopathological examination while 15 cases were negative (biopsy was not available in one smear positive patient).
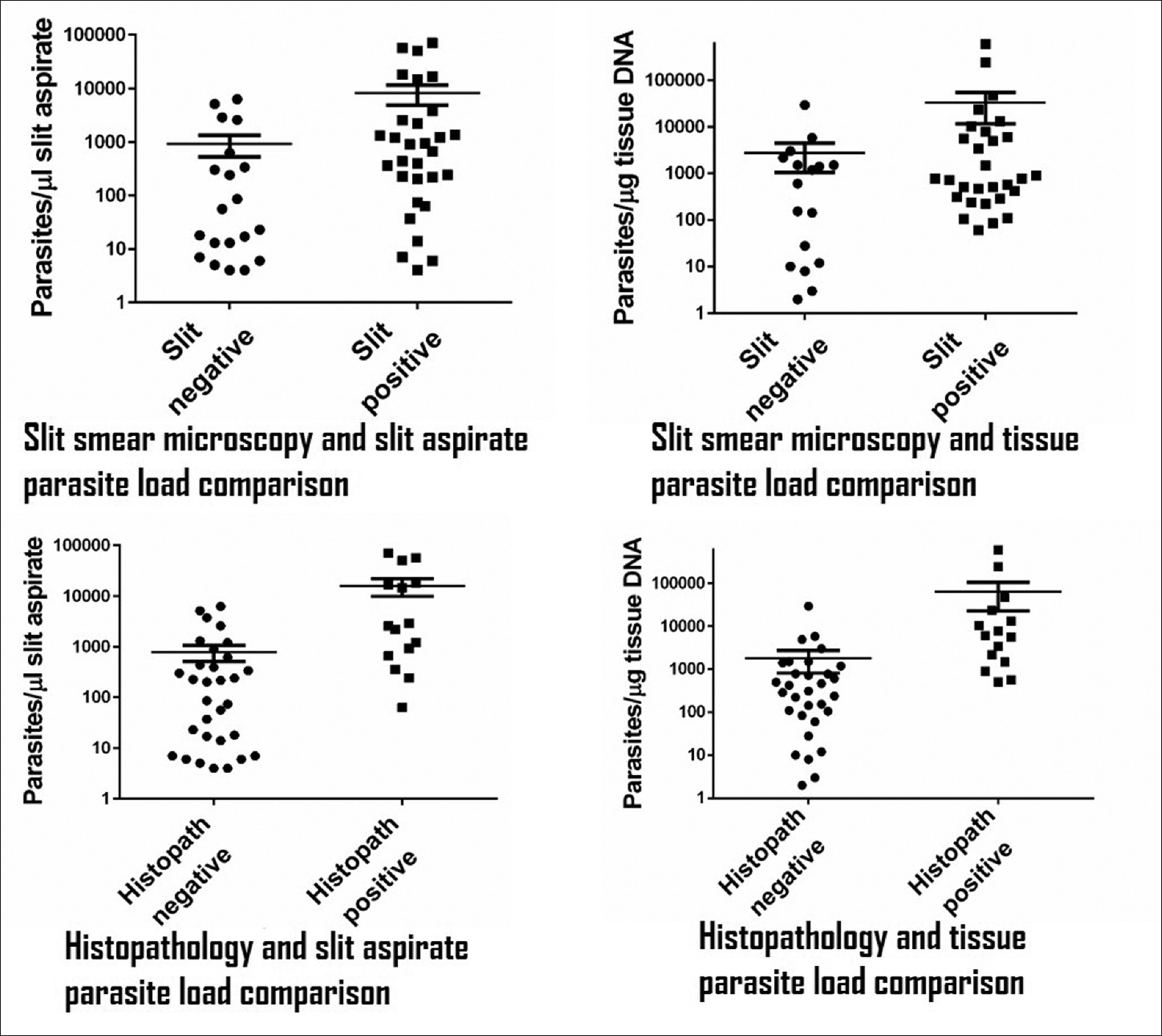 |
| Figure 1: Sensitivity threshold for detection of LD bodies in slit-skin smear and tissue biopsy specimens |
Verma et al. compared the sensitivity of microscopy with the tissue parasite load,[12] and showed that microscopy was positive in >80% cases with high parasite load in tissue biopsy. He also demonstrated lower parasite loads in macules and papules similar to our results.[11]
The slit-skin smear has high specificity, implying that it can confidently identify the uninfected as negative. The morphology of the LD bodies is unique and is unlikely to be confused with other cytological forms. Histopathologically, in some cases, the nuclear debris in phagocytes may mimic amastigotes.[13] However, the sensitivity for diagnosing PKDL is high for molecular tests because the threshold of parasite load for positivity of these tests is significantly lower than that of slit-skin smear and histopathological examination.[12] Immunological tests such as ELISA, direct agglutination test, and rK39 test may not give conclusive results because of the persistence of the Leishmania antibodies for years after infection.[2]
Operational characteristics
The slit skin smear is underutilized by both clinicians and microbiologists in PKDL. It can yield results within an hour, is minimally invasive, and can be performed in resource-poor settings with routine stains and a microscope. It is suitable for children and adults with facial lesions who may be unwilling for biopsy. Technicians can be trained to look for LD bodies. The reagents used are stable at room temperature and need minimum maintenance. The slit-skin smear samples are stable at tropical temperatures and easily transported.
Functionality
The choice of the test varies in different health care settings. Considering the performance and operational characteristics of slit-skin smear, the functionality of the test can be analyzed. For a developed country, highly sensitive and sophisticated tests are appropriate. They may be used in conjunction with direct microscopy or immunological tests.
However, the regions worst affected by PKDL are the less developed nations. Most sophisticated tests available for diagnosis have their own challenges. Lack of resources limit developing countries, and many new tests are marketed directly to end users who lack the skills to assess their performance,[14] leading to misdiagnosis, misinterpretation, and delayed treatment, contributing to poor case detection [Figure - 2].
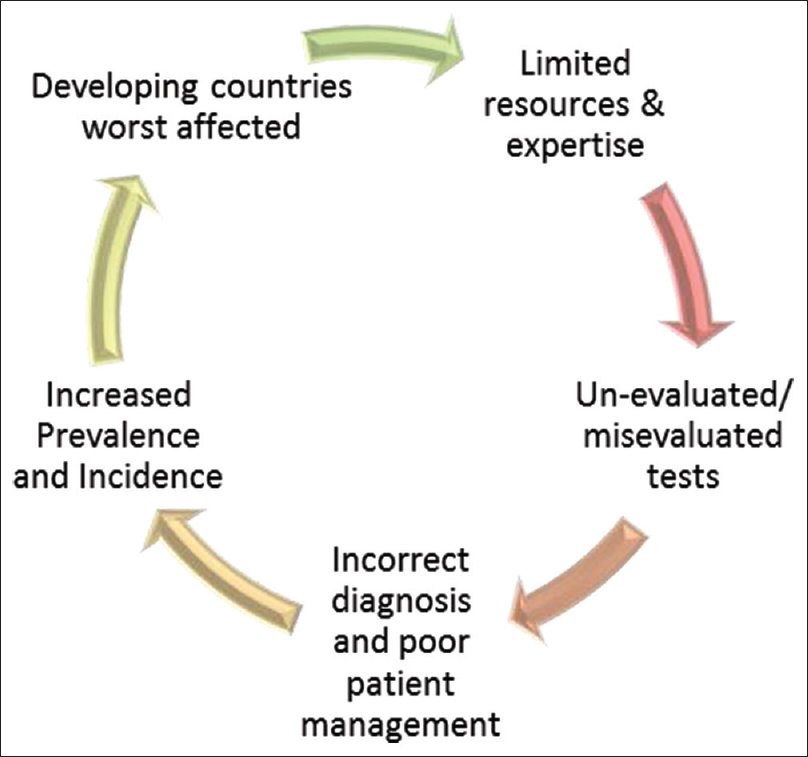 |
| Figure 2: Challenges faced by developing countries |
Factors Influencing the Results in Diagnosing PKDL by Slit Skin Smear Examination
The method of slit skin smear preparation has been described earlier in detail.[12] In brief, an appropriate lesion is selected in which which a slit is made and tissue smears are taken.
Preanalytical variables
- Clinical and epidemiological features: A proper history and examination are important. They not only lead to the provisional diagnosis of PKDL but also support the laboratory findings
- Site of sample collection: A positive result depends upon the type of lesion, with maximum sensitivity in the case of nodules and least for macules
- Sample collection technique: The quantity of tissue pulp retrieved and used to prepare the smear is important because of low parasite load in the lesion. Mixing of blood distorts the morphology of the LD bodies rendering interpretation difficult
- Storage: Unstained smears if not stored properly may gather dust and lead to development of artefacts
- Mislabelling of slide with a wrong name or on the wrong side may lead to improper results.
Analytical variables
- Staining technique: Poor fixing and staining of smear may decrease the yield of the test[15]
- Technique of slit skin smear examination: The technique of smear examination should be such that at least 100 oil immersion fields with adequate cellular components are visualized before declaring the smear negative. This needs a minimum of 10 minutes of zigzag examination.
- Reporting errors include excessive turn-around time, improper data entry, and transcription errors such as any other tests which interfere with the accuracy of results.
Confirming the Presence of LD Bodies
In a slit-skin smear, LD bodies can be seen lying singly or in clusters, either intracellularly within mononuclear macrophages or as extracellular structures. The amastigotes have the following characteristics [Figure - 3]:
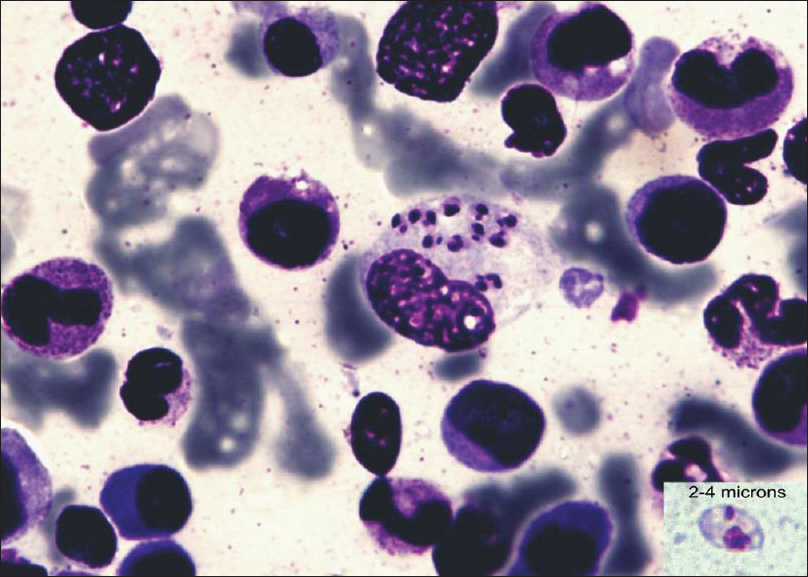 |
| Figure 3: Macrophage with intracellular LD bodies (Giemsa stain, ×1000) and morphology of a typical LD body (in inset) |
- Round or oval body 2–4 μ in size along longitudinal axis
- Presence of a delicate cell membrane[16]
- Presence of a nucleus and kinetoplast lying at right angles to each other
- The nucleus is approximately 5–6 times the size of the kinetoplast
- The color of the kinetoplast and the nucleus is same as that of the nucleus of mononuclear cells in the smear.
On high quality microscopes with a good resolution, a vacuole along the axoneme may be appreciated [Figure - 3] and [Figure - 4].
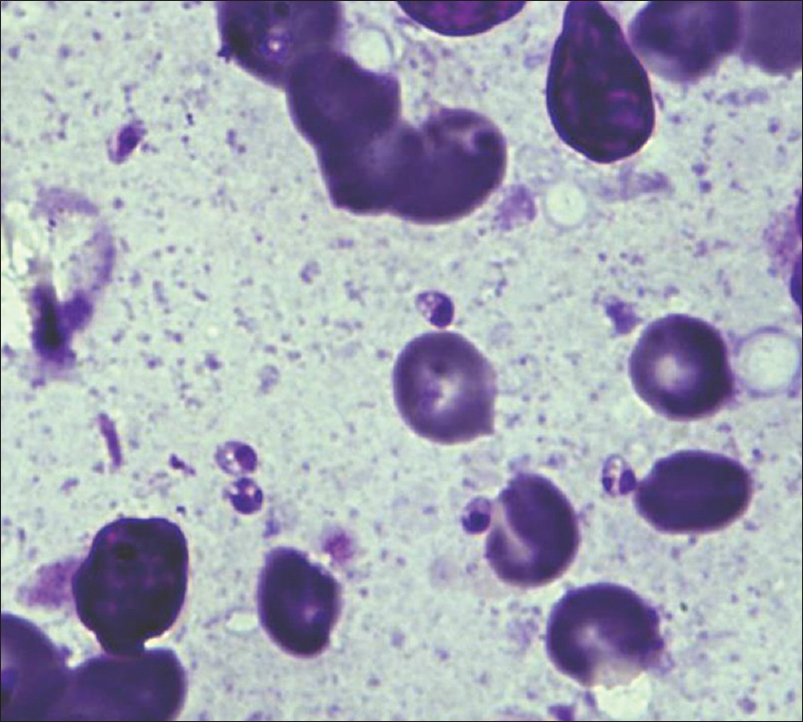 |
| Figure 4: Extra-cellular LD body (Giemsa stain, ×1000) |
The above-mentioned characteristics of LD bodies were analyzed for each slit skin smear examined. The smear positive cases were validated against the findings of PCR. Based upon the analysis and to improve the reliability of slit-skin smear, four scenarios are proposed. The diagnosis of PKDL should be considered when any one is fulfilled [Table - 3]:
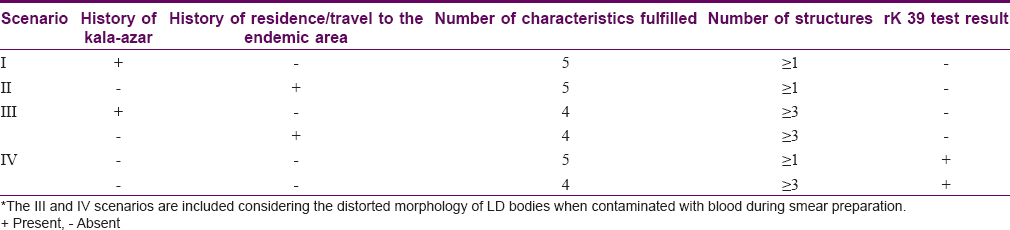
- Scenario I: History of kala-azar and presence of at least one structure fulfilling all the five characteristics
- Scenario II: History of residence/travel to a kala-azar endemic area and presence of at least one structure fulfilling all the five characteristics
- Scenario III: History of kala-azar or residence/travel to an endemic area and presence of atleast 3 or more structures fulfilling minimum 4 characteristics
- If the rapid rk 39 serological test can be performed, then a fourth one can be included
- Scenario IV: A positive rk39 test in serum and/or from tissue aspirate and presence of atleast one structure fulfilling all the five characteristics mentioned above or presence of atleast 3 or more structures fulfilling minimum 4 characteristics.
In cases from nonendemic areas, with no history of kala-azar or residence/travel to an endemic area, the presence of structures meeting the characteristics of LD bodies may be encountered. These scenarios help in diagnosing PKDL in such cases where the smears present with LD bodies with a distorted morphology[Figure - 5]. In such situations, the possibility of cutaneous leishmaniasis or other organisms mimicking LD bodies should be considered. The diagnosis must be confirmed by specific tests.
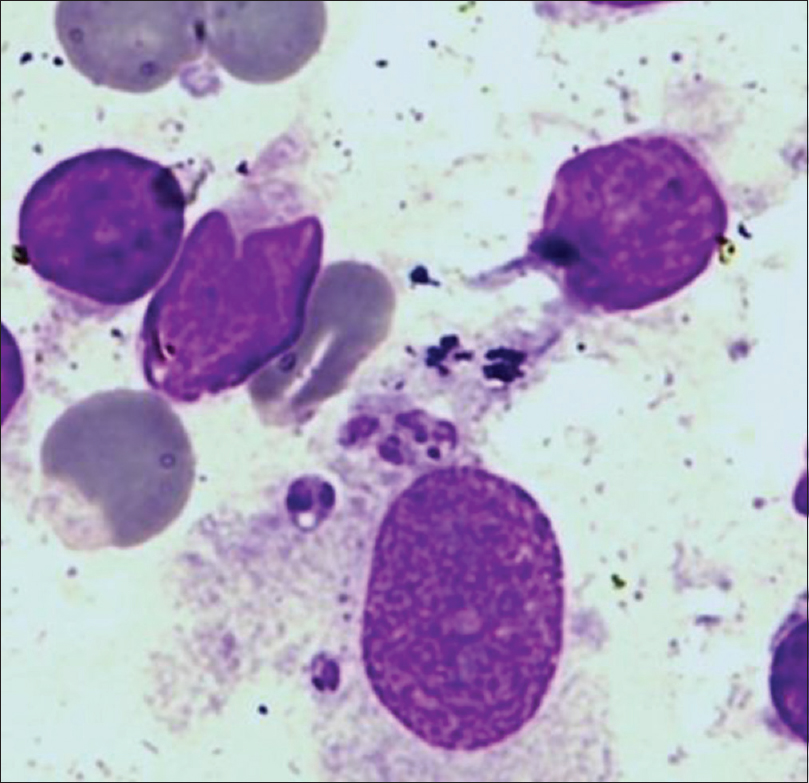 |
| Figure 5: Distorted morphology of LD body in a contaminated sample (Giemsa stain, ×1000) |
Conclusions
- The slit skin smear is a patient-friendly minimally invasive diagnostic method as it is more sensitive and has a lower threshold of detection of LD bodies as compared to histopathological examination
- For the field diagnosis of PKDL in endemic areas, the slit-skin smear can be used as a primary confirmatory test in rk39 positive cases
- All slit-skin smear negative patients clinically suspected as PKDL should be evaluated by molecular methods, if necessary.
Financial support and sponsorship
No financial support was availed for performing the present study.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Ganguly S, Saha P, Chatterjee M, Roy S, Ghosh TK, Guha SK, et al. PKDL – A silent parasite pool for transmission of leishmaniasis in kala-azar endemic areas of Malda District, West Bengal, India. PLoS Negl Trop Dis 2015;9:e0004138.
[Google Scholar]
|
| 2. |
Salotra P, Singh R. Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian J Med Res 2006;123:295-310.
[Google Scholar]
|
| 3. |
Ramesh V, Mukherjee A. Post-kala-azar dermal leishmaniasis. Int J Dermatol 1995;34:85-91.
[Google Scholar]
|
| 4. |
Sharma MC, Gupta AK, Verma N, Das VN, Saran R, Kar SK. Demonstration of leishmania parasites in skin lesions of Indian post kala-azar dermal leishmaniasis (PKDL) cases. J Commun Dis 2000;32:67-8.
[Google Scholar]
|
| 5. |
Verma N, Bimal S, Das VN, Pandey K, Singh D, Lal CS,et al. Clinico pathological and immunological changes in Indian post kala-azar dermal leishmaniasis (PKDL) cases in relation to treatment: A retrospective study. Biomed Res Int 2015;745062:1-5.
[Google Scholar]
|
| 6. |
Zijlstra EE, Khalil EA, Kager PA, El-Hassan AM. Post-kala-azar dermal leishmaniasis in the Sudan: clinical presentation and differential diagnosis. Br J Dermatol 2000;143:136-43.
[Google Scholar]
|
| 7. |
Singh RP. Observation on dermal leishmanoid in Bihar. Indian J Dermatol 1968;13:59-63.
[Google Scholar]
|
| 8. |
Beena KR, Ramesh V, Mukherjee A. Identification of parasite antigen, correlation of parasite density and inflammation in skin lesions of post kala-azar dermal leishmaniasis. J Cutan Pathol 2003;30:616-20.
[Google Scholar]
|
| 9. |
Verma S, Kumar R, Katara GK, Singh LC, Negi NS, Ramesh V, et al. Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One 2010;5:e10107.
[Google Scholar]
|
| 10. |
Bahamdan KA, Khan AR, Tallab TM, Mourad MM. Value of touch preparations (imprints) for diagnosis of cutaneous leishmaniasis. Int J Dermatol 1996;35:558-60.
[Google Scholar]
|
| 11. |
Goihman-Yahr M. American mucocutaneous leishmaniasis. Dermatol Clin 1994;12:703-12.
[Google Scholar]
|
| 12. |
Verma S, Bhandari V, Avishek K, Ramesh V, Salotra P. Reliable diagnosis of post-kala-azar dermal leishmaniasis (PKDL) using slit aspirate specimen to avoid invasive sampling procedures. Trop Med Int Health 2013;18:268-75.
[Google Scholar]
|
| 13. |
Singh A, Ramesh V, Ramam M. Histopathological characteristics of PKDL: a series of 88 patients. Indian J Dermatol Venereol Leprol 2015;81:29-34.
[Google Scholar]
|
| 14. |
TDR Diagnostics Evaluation Expert Panel, Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol 2008;6 11 Suppl:S16-26.
[Google Scholar]
|
| 15. |
Samuel LP, Balada-Llasat JM, Harrington A, Cavagnolo R. Correction for Samuel et al., Multicenter assessment of gram stain error rates. J Clin Microbiol 2016;54:2405.
[Google Scholar]
|
| 16. |
Chaterjee KD. Parasitology: Protozoology and Helminthology. 2014, 13th ed. New Delhi, India, CBS Publishers and Distributors Pvt. Ltd.; 2009. p. 67.
[Google Scholar]
|
Fulltext Views
8,964
PDF downloads
1,575





