Translate this page into:
Risk of skin cancer in kidney, liver and heart recipients: A nationwide population-based study in Taiwan
-
Received: ,
Accepted: ,
How to cite this article: Liu S-W, Wang W-M, Chiang C-P, Chung C-H, Tsao C-H, Chien W-C, et al. Risk of skin cancer in kidney, liver and heart recipients: A nationwide population-based study in Taiwan. Indian J Dermatol Venereol Leprol 2023;89:372-7.
Abstract
Background
Previous population-based studies in western countries had revealed increased skin cancer risk among transplant recipients compared to the general population. However, population-based studies in Asia on skin cancer among recipients of different transplanted organs were lacking in the literature.
Aims
This study aims to estimate skin cancer risk among recipients in Taiwan, examine the association between each specific type of skin cancer and each type of transplanted organ, and compare skin cancer risk between different immunosuppressive regimens.
Methods
This population-based retrospective cohort study identified 7550 patients with heart, lung, kidney or liver transplantation and 30,200 controls matched for gender, age and comorbidity index from the National Health Insurance Research Database in Taiwan between 2000 and 2015. Using multivariable Cox proportional hazard models, we estimated the hazard ratios and 95% confidence intervals for the correlation of skin cancer with organ transplantation as well as immunosuppressive regimen.
Results
Organ transplant recipients in Taiwan had an increased risk of skin cancer with adjusted hazard ratios of 4.327 (95% confidence intervals 2.740-6.837, P < 0.001), with the greatest risk, observed among heart recipients (adjusted hazard ratios 6.348, 95% confidence intervals 3.080-13.088, P < 0.001). The risk of non-melanoma skin cancer and melanoma was 4.473 (95% confidence intervals 2.568-7.783, P < 0.001) and 3.324 (95% confidence intervals 1.300-8.172, P < 0.001), respectively. When comparing immunosuppressants, those with calcineurin inhibitors carried the highest risk of skin cancer (adjusted hazard ratios 4.789, 95% confidence intervals 3.033-7.569, P < 0.001), followed by those with antimetabolites (adjusted hazard ratios 4.771, 95% confidence intervals 3.025-7.541, P < 0.001).
Limitations
We could not evaluate confounding behavioural risk factors of skin cancers that were not documented in the database, nor could we recognize patients’ compliance with immunosuppressants.
Conclusion
Organ recipients have a greater risk of skin cancer. Clinicians should inform recipients of the importance of photoprotection and regular dermatologic follow-up.
Keywords
Kaposi’s sarcoma
melanoma
non-melanoma skin cancer
organ transplant
skin cancer
Plain Language Summary
This population-based retrospective cohort study identified 7550 patients with heart, lung, kidney or liver transplantation and 30,200 controls matched for gender, age and comorbidity index from the National Health Insurance Research Database in Taiwan between 2000 and 2015. Organ transplant recipients had a 4.327-fold higher risk of skin cancer, with heart recipients carrying the highest risk. The risk of non-melanoma skin cancer and melanoma was 4.473 and 3.324, respectively. When comparing immunosuppressants, those treated with calcineurin inhibitors carried the highest risk. Clinicians should inform transplant recipients of the importance of sun protection and regular dermatologic follow-up.
Introduction
As the survival of organ recipients increased over the past decades, the long-term complications due to post-transplant immunosuppressive therapy had become more prevalent.1 Previous population-based studies in western countries had revealed increased skin cancer risk among recipients compared to the general population,2-4 as had a Korean single-centre cohort study in 2014.5 However, the Korean study did not evaluate the risk of each specific type of skin cancer among recipients with different transplanted organs. In 2016, a Taiwan population-based cohort study on cancer risk among transplant recipients demonstrated no significant association between transplantation and skin cancer.6
We conducted a nationwide cohort study in Taiwan to estimate the risk of skin cancer among transplant recipients. We additionally analyzed the risk of each specific type of skin cancer among those with different transplanted organs and compared skin cancer risk between different immunosuppressive regimens, both of which are currently lacking in the literature.
Method
Data sources
This retrospective cohort study was based on data acquired from National Health Insurance Research Database in Taiwan. The National Health Insurance Program, established in 1995, is a mandatory single-payer system enrolling more than 99% of the population, i.e. approximately 23 million residents of Taiwan. National Health Insurance Research Database comprises original claims data for reimbursement from ambulatory care and inpatient care and provides de-identified information about individuals’ age, sex, dates of visits, diagnostic codes designated by the International Classification of Disease, Revision 9, Clinical Modification and treatments. The study was reviewed and approved by the Institutional Review Board (TSGHIRB Number: No.C202005083).
Study participants
Using National Health Insurance Research Database data, we identified patients with heart (OP37.5), liver (OP50.5), kidney (OP55.6), lung (OP33.5) and combined heart-lung transplantation (OP33.6) between 2000 and 2015. Patients were tracked from the date of transplantation until the date of the first-time diagnosis of skin cancer, withdrawal from the National Health Insurance program, or the end of 2015. Cases of skin cancer were identified by using the International Classification of Disease, Revision 9, Clinical Modification code of 172 for melanoma of the skin, 173 for non-melanoma skin cancer, and 176.0 for Kaposi’s sarcoma of the skin. The exclusion criteria included age younger than 20 years old, unknown gender, transplantation before the index date, multi-organ transplantation, skin cancers before tracking or within 30 days after transplantation and loss to follow-up.
We defined the control group to be matched with the study group for age, gender, index year and comorbidity index. In addition, we excluded those taking a calcineurin inhibitor (cyclosporine and tacrolimus), antimetabolite (azathioprine and mycophenolate mofetil) or mammalian target of rapamycin (mTOR) inhibitor (sirolimus and everolimus) in the control group, in order to compare the hazard ratios for skin cancer among recipients on different immunosuppressive regimens. A total of 7,550 and 30,200 patients were enrolled in the study group and the control group, respectively [Figure 1].

- The flowchart of study sample selection
Outcome measures
Covariates
The covariates encompassed gender, age, age groups (20-39, 40-59, ≥60 years), comorbidities, level of hospital, seasons, level of urbanisation and location of residence. Comorbidities were evaluated by the Charlson Comorbidity Index revised. Charlson Comorbidity Index classifies comorbidities into several categories, scores each category and sums up all scores.7 The higher score indicates the more severe comorbidity. We defined the Charlson Comorbidity Index revised as Charlson Comorbidity Index excluding skin cancer since patients with skin cancer before tracking was already excluded in both the study and the control group.
Statistical analysis
All the data were analysed by SPSS software version 22 (SPSS Inc., Chicago, Ill., USA). The prevalence (per 105 person-years) was calculated as the number of patients with the incident skin cancer divided by the total person-years of each group. We used multivariable Cox proportional hazard models to estimate hazard ratios and 95% confidence intervals.The Kaplan-Meier method was used to plot the cumulative risk of skin cancer, and a log-rank test was used to compare the two cohorts. A 2-tailed P value <0.05 and a confidence interval not crossing one indicates statistical significance.
Results
Clinical characteristics of patients and controls
This cohort study included 7550 patients with organ transplants and 30,200 controls with neither organ transplant nor immunosuppressive therapy between 2000 and 2015. There were no differences in gender, age, age group and comorbidities between the two groups [Table 1]. The mean follow-up time was 10.12 ± 9.27 years.
| Transplantation | Total | With | Without | |||
|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % |
| Total | 37,750 | 7550 | 20.00 | 30,200 | 80.00 | |
| Gender | ||||||
| Male | 23,925 | 63.38 | 4785 | 63.38 | 19,140 | 63.38 |
| Female | 13,825 | 36.62 | 2765 | 36.62 | 11,060 | 36.62 |
| Age (years) | 48.02 ± 12.45 | 48.04 ± 11.16 | 48.02 ± 12.75 | |||
| Age groups (years) | ||||||
| 20-39 | 8975 | 23.77 | 1795 | 23.77 | 7180 | 23.77 |
| 40-59 | 23,515 | 62.29 | 4703 | 62.29 | 18,812 | 62.29 |
| ≥60 | 5260 | 13.93 | 1052 | 13.93 | 4208 | 13.93 |
| CCI_R | 1.74 ± 1.52 | 1.76 ± 1.54 | 1.73 ± 1.51 | |||
CCI_R: Charlson comorbidity index revised, presented as Mean ± Standard deviation (SD); Age (years), presented as Mean ± SD
Hazard ratios analysis of skin cancer among patients with an organ transplant
With age, gender, comorbidity, season, location, urbanisation level, and level of care adjusted, patients with organ transplants were found to have a greater risk of skin cancer (adjusted hazard ratios 4.327, 95% confidence intervals 2.740-6.837, P < 0.001, Table 2). Subgroup analysis revealed that recipients in all age groups had significantly higher skin cancer risk than the controls (P < 0.001). In addition, elevated skin cancer risk among recipients was identified regardless of gender (P < 0.001).
| Transplantation | With | Without (Reference) | With vs. Without (Reference) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | PYs | Prevalence | Events | PYs | Prevalence | Adjusted HR | 95% CI | P |
| Total | 36 | 72,900.37 | 49.38 | 64 | 311,233.70 | 20.56 | 4.327 | 2.740-6.837 | <0.001 |
| Gender | |||||||||
| Male | 24 | 41,449.63 | 57.90 | 45 | 198,775.63 | 22.64 | 4.609 | 2.918-7.289 | <0.001 |
| Female | 12 | 31,450.74 | 38.15 | 19 | 112,458.07 | 16.90 | 4.072 | 2.542-6.433 | <0.001 |
| Age groups (yrs) | |||||||||
| 20-39 | 3 | 11,548.99 | 25.98 | 6 | 51,216.22 | 11.72 | 3.983 | 2.820-6.304 | <0.001 |
| 40-59 | 15 | 40,753.87 | 36.81 | 22 | 139,381.23 | 15.78 | 4.197 | 2.648-3.326 | <0.001 |
| ≥60 | 18 | 20,597.50 | 87.39 | 36 | 120,636.25 | 29.84 | 5.268 | 3.334-8.327 | <0.001 |
Adjusted HR: Adjusted hazard ratio, adjusted with age, gender, comorbidity, season, location, urbanisation level, and level of care; CI: Confidence interval, PYs: Person-years
Skin cancer type and transplanted organ
The risk of overall skin cancer was the highest among heart recipients (adjusted hazard ratios 6.348, 95% confidence intervals 3.080-13.088, P < 0.001, Table 3), followed by liver (adjusted hazard ratios 4.987, 95% confidence intervals 2.590-9.762, P < 0.001), and kidney (adjusted hazard ratios 2.717, 95% confidence intervals 1.518-5.024, P < 0.001) recipients.
| Events subgroups | Transplantation subgroups | Populations | Events | PYs | Prevalence | Adjusted HR | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| Skin cancer | Without transplantation | 30,200 | 64 | 311,233.70 | 20.56 | Reference | ||
| With transplantation | 7550 | 36 | 72,900.37 | 49.38 | 4.327 | 2.740-6.837 | <0.001 | |
| Lung | 72 | 0 | 572.52 | 0.00 | 0.000 | - | 0.987 | |
| Combined heart-lung | 2 | 0 | 0.01 | 0.00 | 0.000 | - | 0.999 | |
| Heart | 878 | 9 | 11,186.76 | 80.45 | 6.348 | 3.080-13.088 | <0.001 | |
| Liver | 3137 | 12 | 18,355.59 | 65.38 | 4.987 | 2.590-9.762 | <0.001 | |
| Kidney | 3461 | 15 | 42,785.48 | 35.06 | 2.717 | 1.518-5.024 | <0.001 | |
| NMSC | Without transplantation | 30,200 | 43 | 311,233.70 | 13.82 | Reference | ||
| With transplantation | 7550 | 24 | 72,900.37 | 32.92 | 4.473 | 2.568-7.783 | <0.001 | |
| Lung | 72 | 0 | 572.52 | 0.00 | 0.000 | - | 0.986 | |
| Combined heart-lung | 2 | 0 | 0.01 | 0.00 | 0.000 | - | 0.999 | |
| Heart | 878 | 7 | 11,186.76 | 62.57 | 7.677 | 3.373-17.968 | <0.001 | |
| Liver | 3137 | 9 | 18,355.59 | 49.03 | 5.515 | 2.495-13.262 | <0.001 | |
| Kidney | 3461 | 8 | 42,785.48 | 18.70 | 2.098 | 0.945-4.786 | 0.080 | |
| Malignant melanoma of skin | Without transplantation | 30,200 | 19 | 311,233.70 | 6.10 | Reference | ||
| With transplantation | 7550 | 8 | 72,900.37 | 10.97 | 3.324 | 1.300-8.172 | 0.006 | |
| Lung | 72 | 0 | 572.52 | 0.00 | 0.000 | - | 0.998 | |
| Combined heart-lung | 2 | 0 | 0.01 | 0.00 | 0.000 | - | 0.999 | |
| Heart | 878 | 2 | 11,186.76 | 17.88 | 4.983 | 1.195-25.047 | 0.010 | |
| Liver | 3137 | 1 | 18,355.59 | 5.45 | 0.985 | 0.195-10.833 | 0.672 | |
| Kidney | 3461 | 5 | 42,785.48 | 11.69 | 3.192 | 1.062-9.499 | 0.039 | |
| Kaposi sarcoma of skin | Without transplantation | 30,200 | 2 | 311,233.70 | 0.64 | Reference | ||
| With transplantation | 7550 | 4 | 72,900.37 | 5.49 | 12.937 | 2.183-79.714 | <0.001 | |
| Lung | 72 | 0 | 572.52 | 0.00 | 0.000 | - | 0.999 | |
| Combined heart-lung | 2 | 0 | 0.01 | 0.00 | 0.000 | - | 0.999 | |
| Heart | 878 | 0 | 11,186.76 | 0.00 | 0.000 | - | 0.999 | |
| Liver | 3137 | 2 | 18,355.59 | 10.90 | 17.677 | 1.990-171.324 | <0.001 | |
| Kidney | 3461 | 2 | 42,785.48 | 4.67 | 14.252 | 1.555-131.898 | 0.002 |
Adjusted HR: Adjusted hazard ratio, adjusted with age, gender, comorbidity, season, location, urbanisation level, and level of care; CI: Confidence interval, PYs: Person-years, NMSC: Non-melanoma skin cancer
Non-melanoma skin cancer was positively correlated with heart (adjusted hazard ratios 7.677, 95% confidence intervals 3.373-17.968, P < 0.001) and liver (adjusted hazard ratios 5.515, 95% confidence intervals 2.495-13.262, P < 0.001) recipients, but not kidney recipients (adjusted hazard ratios 2.098, 95% confidence intervals 0.945-4.786, P = 0.080).
For melanoma, an elevated risk was observed among heart (adjusted hazard ratios 4.983, 95% confidence intervals 1.195-25.047, P = 0.010) and kidney (adjusted hazard ratios 3.192, 95% confidence intervals 1.062-9.499, P = 0.039) recipients. However, no significant association was found between melanoma and liver recipients (adjusted hazard ratios 0.985, 95% confidence intervals 0.195-10.833, P = 0.672).
Kaposi’s sarcoma developed in two out of the 30,200 controls, and in four out of the 7550 recipients. Due to the rarity of Kaposi’s sarcoma, we additionally analyzed the data by using Poisson regression, and a significant higher risk among recipients was observed (P = 0.005).
Hazard ratios analysis of skin cancer among patients on different immunosuppressants
We recorded all anti-rejection agents prescribed for the enrolled transplant recipients before tracking endpoint and categorised these agents into six groups [Table 4]. All six groups of immunosuppressive regimens significantly increased the risk of skin cancers, with calcineurin inhibitors (adjusted hazard ratios 4.789, 95% confidence intervals 3.033-7.569, P < 0.001) carrying the highest risk, followed by antimetabolites (adjusted hazard ratios 4.771, 95% confidence intervals 3.025-7.541, P < 0.001), mTOR inhibitors (adjusted hazard ratios 4.75, 95% confidence intervals 3.007-7.511, P < 0.001), calcineurin inhibitor combined with antimetabolite (adjusted hazard ratios 4.362, 95% confidence intervals 2.768-6.906, P < 0.001), calcineurin inhibitor combined with mTOR inhibitor (adjusted hazard ratios 3.884, 95% confidence intervals 2.459-6.135, P < 0.001), and antimetabolite combined with mTOR inhibitor (adjusted hazard ratios 3.543, 95% confidence intervals 2.245-5.598, P < 0.001). The lowest risk of skin cancer was observed among those on the combination of calcineurin inhibitor, antimetabolite and mTOR inhibitor (adjusted hazard ratios 2.839, 95% confidence intervals 1.796-4.482, P < 0.001).
| Anti-rejection agent subgroups | Populations | Events | PYs | Prevalence | Adjusted HR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Without transplantation | 30,200 | 64 | 311,233.70 | 20.56 | Reference | ||
| With transplantation | 7550 | 36 | 72,900.37 | 49.38 | 4.327 | 2.740-6.837 | <0.001 |
| Calcineurin inhibitor only | 1328 | 7 | 12,812.66 | 54.63 | 4.789 | 3.033-7.569 | <0.001 |
| Antimetabolite only | 1864 | 10 | 18,362.45 | 54.46 | 4.771 | 3.025-7.541 | <0.001 |
| mTOR inhibitor only | 1135 | 6 | 11,062.14 | 54.24 | 4.750 | 3.007-7.511 | <0.001 |
| Calcineurin inhibitor + Antimetabolite | 826 | 4 | 8,026.35 | 49.84 | 4.362 | 2.768-6.906 | <0.001 |
| Calcineurin inhibitor + mTOR inhibitor | 938 | 4 | 9,034.11 | 44.28 | 3.884 | 2.459-6.135 | <0.001 |
| Antimetabolite + mTOR inhibitor | 767 | 3 | 7,421.09 | 40.43 | 3.543 | 2.245-5.598 | <0.001 |
| Calcineurin inhibitor + Antimetabolite + mTOR inhibitor | 692 | 2 | 6,181.57 | 32.35 | 2.839 | 1.796-4.482 | <0.001 |
Adjusted HR: Adjusted hazard ratio, adjusted with age, gender, comorbidity, season, location, urbanisation level, and level of care; CI: Confidence interval, PYs: Person-years, mTOR: mammalian target of rapamycin
Kaplan-Meier model for the cumulative risk of skin cancer
The Kaplan-Meier analysis indicated that the cumulative risk of non-melanoma skin cancer had been higher since the seventh year (P = 0.033, Figure 2), and the risk of melanoma had been higher since the eleventh year post-transplantation (P = 0.046).
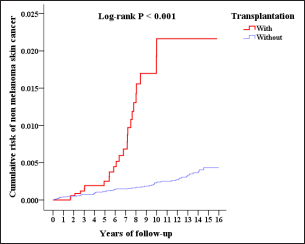
- Cumulative risk of non-melanoma skin cancer of population-based Taiwan cohort (2000-2015)
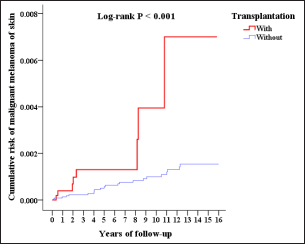
- Cumulative risk of malignant melanoma of skin of population-based Taiwan cohort (2000-2015)
Discussion
The results of this nationwide population-based cohort study revealed that organ transplant recipients had an increased risk of skin cancer with an adjusted hazard ratio of 4.327 (P <0.001). In 2012, a Swedish nationwide cohort study of 10,476 organ transplant recipients revealed an 83-fold, 54-fold, and 16-fold higher risk of skin cancer among patients with heart, kidney, and liver transplants, respectively, compared with the general population.3 In Asia, a Korean single-centre based cohort study in 2013 enrolling 4444 transplant recipients found that the standardised incidence ratio of skin cancer among recipients was 30.9 after the fifth post-transplantation year.5 In contrast, a previous population-based cohort study of 5396 transplant recipients in Taiwan in 2016 showed that neither heart, kidney, nor liver recipients had significantly increased skin cancer risk compared to the general population.6
Among 3461 kidney recipients, two patients got Kaposi’s sarcoma, and we observed an increased risk of melanoma with an adjusted hazard ratio of 3.192. In the aforementioned Swedish study, the standardised incidence ratios of melanoma, Kaposi’s sarcoma and squamous cell carcinoma of skin were 2.3, 40 and 121, respectively, among kidney recipients.3 Most patients waiting for kidney transplantation had uremia, a state of chronic inflammation8 and immune dysfunction which leads to carcinogenesis.9 An Australian population-based cohort study compared cancer incidence rates in patients with end-stage kidney disease before and after transplantation and revealed that patients had a significantly higher risk of Kaposi sarcoma than the general population during dialysis. The risk further increased after transplantation, indicating both pre-existing uremic status and post-transplant immunosuppressive therapy contributed to cancer development.10 Fitzpatrick skin type was found to be an independent risk factor of squamous cell carcinoma of skin among transplant recipients.11 In contrast to previous studies in the Netherlands and Sweden,3,12 we did not find a significantly higher risk of non-melanoma skin cancer among kidney recipients, possibly related to darker skin of Taiwanese compared to the westerners.
Among liver recipients, our study revealed an elevated risk of non-melanoma skin cancer with an adjusted hazard ratio of 5.515. In the Swedish cohort study, the standardised incidence ratio of squamous cell carcinoma was 32 among liver recipients, whereas the standardised incidence ratio of melanoma did not show statistical significance (0.3-4.5).3 Eradication of hepatitis B virus and human papillomavirus infections involve a shared immune response.13 Hepatitis B virus patients with defects in the immune system are at increased risk of concomitant human papillomavirus infection, which could contribute to skin cancer. No significant difference in the risk of melanoma was observed among liver recipients in our study (adjusted hazard ratios 0.985, P = 0.672).
A Norwegian cohort study in 1999 found that heart recipients carried a 2.9-fold higher risk of cutaneous squamous cell carcinoma than kidney recipients.14 The aforementioned Swedish and Korean studies both observed a higher skin cancer risk among heart recipients than kidney or liver recipients.3,5 Consistently, our study revealed that heart recipients had the greatest risk (adjusted hazard ratios 6.348) of skin cancers compared to the liver (adjusted hazard ratios 4.987) or kidney (adjusted hazard ratios 2.717) recipients. A higher dosage of immunosuppressants required for heart recipients was considered responsible for this finding.
The pathogenesis of skin cancer in recipients involves photocarcinogenesis due to ultraviolet radiation, oncogenic viruses (human papillomavirus and human herpesvirus 8, etc.), host genetic susceptibility, and most importantly, long-term immunosuppressive treatment.15 However, only a few studies had compared skin cancer risk between different immunosuppressive agents. Our study revealed that all the six groups of immunosuppressive regimens were significantly related to increased skin cancer risk, with calcineurin inhibitors (adjusted hazard ratios 4.789, P <0.001) carrying the highest risk. A retrospective cohort study in Spain disclosed lower skin cancer risk among liver recipients on mycophenolate mofetil monotherapy over a calcineurin inhibitor-based regimen.16 Conversion from calcineurin inhibitor to sirolimus reduced the risk of squamous cell carcinoma and non-melanoma skin cancer as separately demonstrated by two randomized controlled trials.17,18 In addition to their immunosuppressive effect, calcineurin inhibitors and azathioprine exert photocarcinogenesis through disrupting nucleotide excision repair or damaging DNA by generating reactive oxygen species upon UVA contact, respectively.19,20 The lowered risk among patients on mTOR inhibitors could be attributed to the antineoplastic activity of sirolimus via offsetting the activation of the mTOR1 pathway brought by human papillomavirus E6 oncoprotein and inhibiting the PI3K-AKT pathway, which has a crucial role in cell proliferation and cancer progression.21,22 In addition, we speculate that patients on a combined regimen of calcineurin inhibitors, antimetabolite and mTOR inhibitor received a reduced dosage of each. The diminished dose and the antineoplastic activity of the mTOR inhibitor both may have contributed to the lowest risk.
Limitations
Our study is population-based and used National Health Insurance Research Database with its quality guaranteed by the National Health Insurance Authority and government regulation. The method of 4-fold propensity score matching gender, age and comorbidities between the study and comparison cohorts strengthen the statistical power of our study to evaluate the relationship between skin cancer and organ transplantation. Nevertheless, there were several limitations. First, we lacked the information not routinely documented in a claim database about potential behavioural risk factors of skin cancer, such as lifestyle and sun exposure. Second, we could not recognize patients’ compliance with immunosuppressants. Lastly, we did not evaluate systemic corticosteroid in our study, because we were unable to confirm whether corticosteroid was prescribed for anti-rejection treatment or not.
Conclusion
In conclusion, this study demonstrated that organ recipients in Taiwan have a 4.327-fold greater risk of skin cancer. Among different immunosuppressive regimens, patients on calcineurin inhibitor monotherapy had the highest risk (adjusted hazard ratios 4.789, P < 0.001). Clinicians should inform recipients about the potential risk of skin cancers and educate them about the importance of photoprotection as well as regular dermatologic follow-up.
Declaration of patient consent
Institutional Review Board (IRB) permission was obtained for the study.
Financial support and sponsorship
This study was supported by grants from Tri-Service Hospital Research Foundation (TSGH-E111201, and TSGH-B-111018).
Conflicts of interest
There are no conflicts of interest.
References
- Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681-91.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-9.
- [PubMed] [Google Scholar]
- Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008-A Swedish population-based study. Int J Cancer. 2013;132:1429-38.
- [CrossRef] [PubMed] [Google Scholar]
- Nonmelanoma skin cancer frequency and risk factors in Australian heart and lung transplant recipients. JAMA Dermatol. 2019;155:716-719.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of primary skin cancer after organ transplantation: An 18-year single-center experience in Korea. J Am Acad Dermatol. 2014;70:465-72.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer incidence among heart, kidney, and liver transplant recipients in Taiwan. PLoS One. 2016;11:e0155602.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the Charlson comorbidity index using Canadian administrative databases: A perspective on risk adjustment in critical care research. J Crit Care. 2005;20:12-9.
- [CrossRef] [PubMed] [Google Scholar]
- The microinflammatory state in uremia: Causes and potential consequences. J Am Soc Nephrol. 2001;12:1549-57.
- [CrossRef] [PubMed] [Google Scholar]
- Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255-65.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823-31.
- [CrossRef] [PubMed] [Google Scholar]
- Fitzpatrick skin phototype is an independent predictor of squamous cell carcinoma risk after solid organ transplantation. J Am Acad Dermatol. 2013;68:585-591.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506-9.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic hepatitis B infection and non-hepatocellular cancers: A hospital registry-based, case-control study. PLoS One. 2018;13:e0193232.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177-186.
- [CrossRef] [PubMed] [Google Scholar]
- The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1217-224.
- [CrossRef] [PubMed] [Google Scholar]
- Conversion from calcineurin inhibitor-based immunosuppression to mycophenolate mofetil in monotherapy reduces risk of de novo malignancies after liver transplantation. Ann Transplant. 2017;22:141-47.
- [CrossRef] [PubMed] [Google Scholar]
- Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-39.
- [CrossRef] [PubMed] [Google Scholar]
- Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation. 2011;92:303-10.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer in organ transplant recipients: Effects of immunosuppressive medications on DNA repair. Exp Dermatol. 2012;21:2-6.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871-4.
- [CrossRef] [PubMed] [Google Scholar]
- mTOR, cancer and transplantation. Am J Transplant. 2008;8:2212-8.
- [CrossRef] [PubMed] [Google Scholar]
- The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398-407.
- [CrossRef] [PubMed] [Google Scholar]






