Translate this page into:
Ritlecitinib rescues exacerbated vitiligo during the JAK1 inhibitor therapy: More than a coincidence?
Corresponding author: Dr. Chao Ji, Department of Dermatology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China. jichaofy@fjmu.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Tong Z, Wu Z, Zeng X, Huang F, Gong T, Ji C. Ritlecitinib rescues exacerbated vitiligo during the JAK1 inhibitor therapy: More than a coincidence? Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_348_2024
Dear Editor,
Recently, JAK inhibitors demonstrated promising effects in vitiligo treatment by targeting the IFN-γ-CXCL10 cytokine pathway, therefore reducing the production of CXCL10. 1-3 Moreover, a case-control study revealed the significant overexpression of JAK1 and JAK3 in the skin of vitiligo patients compared to normal control, demonstrating the potential effect of JAK1 and JAK3 inhibitors in treating vitiligo.4 Based on the current JAK expression profile observed in vitiligo, it seems that targeting JAK1 and JAK3 might be a potential treatment option for vitiligo. However, we present here a patient with exacerbated vitiligo after starting abrocitinib (a JAK1 inhibitor) for severe atopic dermatitis (AD) and with effective improvement after switching the therapy to ritlecitinib (a JAK3 inhibitor).
A 67-year-old male patient with severe AD presented to our department accompanied by white patches spreading over his back. Physical examination revealed several white patches with irregular shapes and blurred margins distributed across his chest and back. Additionally, pruritic erythematous patches with scales on both the trunk and extremities were noted: Eczema Area and Severity Index (EASI) = 26.4; Peak Pruritus Numerical Rating Scale (PP-NRS) = 9 [Figures 1a and 1b]. Laboratory test results showed a significant elevation in both immunoglobulin E (IgE) and eosinophil count. After excluding all the contraindications of JAK1 inhibitor and obtaining complete informed consent, treatment began with abrocitinib 100 mg daily; meanwhile, he was advised to enhance skin care. Pruritus disappeared within 24 hours after initiating abrocitinib (PP-NRS = 0) and lesions showed continuous improvement over the four months. Unexpectedly, although abrocitinib rapidly improved the symptom of AD, the existing vitiligo lesions expanded and depigmentation progressed, the white patches expanded rapidly and became clinically conspicuous, especially on his face [Figure 1c and 1d] (Total-Vitiligo Area Scoring Index [T-VASI] = 35; Facial-Vitiligo Area Scoring Index [F-VASI] = 16) [Table 1]. On suspicion of vitiligo exacerbated by abrocitinib, we performed a Wood lamp examination, revealing fluorescent achromatic patches, and a biopsy on his back demonstrated the absence of melanocytes in the basal layer of the epidermis along with perivascular infiltration of lymphocytes in the dermis. Besides, the Naranjo Adverse Drug Reaction Probability Scale yielded a score of 4, indicating a possible relationship between the vitiligo exacerbation and the use of abrocitinib. Thus, the JAK1 inhibitor was discontinued. Meanwhile, considering the potential effect of targeting JAK3 in vitiligo lesions, the patient switched the therapy from abrocitinib to ritlecitinib, a JAK3 inhibitor. At the two-month follow-up visit, the physical examination suggested that no new white patches appeared and there was repigmentation on his face and trunk (T-VASI = 28; F-VASI = 5). Additionally, no recurrence of AD lesions has occurred [Figures 1e and 1f] and the patient is still under follow-up [Figure 2].
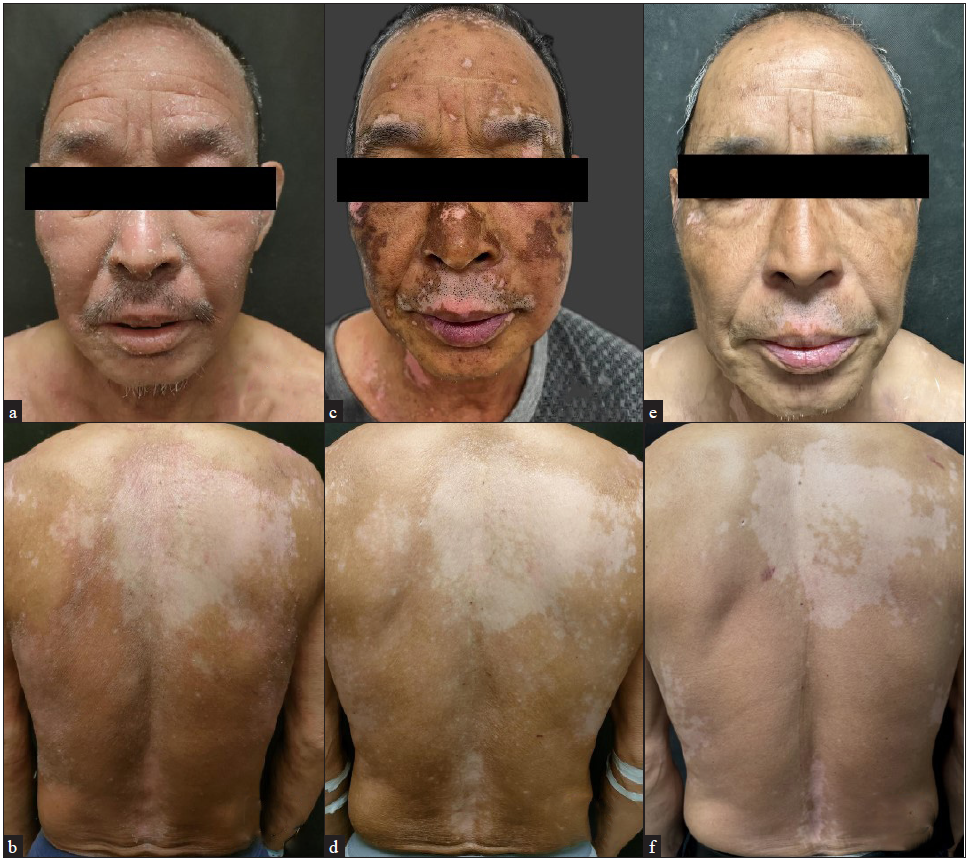
- Clinical photographs of the patient during the follow-up (a,b) before the initiation of the treatments, (c,d) after 1 month treatment of abrocitnib, (e,f) after 2 month treatment of ritlecitinib.
|
Scores Time |
Scores of atopic dermatitis | Scores of vitiligo | ||
|---|---|---|---|---|
| EASI score | PP-NRS score | T-VASI score | F-VASI score | |
| 2023.10.12 | 26.2 | 9 | 20 | 2 |
| 2023.11.12 | 9.2 | 1 | 35 | 16 |
| 2024.01.09 | 7.2 | 2 | 28 | 5 |
EASI: Eczema Area and Severity Index; PP-NRS: Eczema Area and Severity Index; T-VASI: Total-Vitiligo Area Scoring Index; F-VASI: Facial-Vitiligo Area Scoring Index.
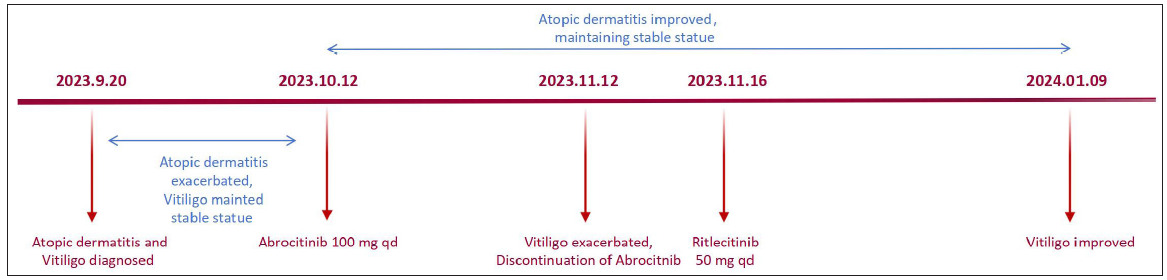
- The timeline of the disease course.
Given recent advancements in our understanding of vitiligo pathogenesis, JAK1 inhibitors are increasingly recognised as promising therapeutic candidates.5 To our best knowledge, our case appears to be the first report in the literature of exacerbated vitiligo during the therapy of JAK1 inhibitor. Notably, the curative effect was observed after the administration of JAK3 inhibitor, vitiligo stopped progressing after treatment and gradually repigmented. This observation suggested that diverse factors may interplay together to affect the clinical outcome of this disease. Despite the upregulation of JAK1 and JAK3 observed in the skin of active vitiligo patients, the imbalanced expression of JAK may contribute to the variation in therapeutic efficacy among patients receiving JAK inhibitors. While the molecular drivers of exacerbated vitiligo occurring during JAK1 inhibitor treatment remain unclear, further research is needed to elucidate the mechanism of vitiligo progression in patients undergoing JAK1 inhibitor therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Tofacitinib citrate for the treatment of vitiligo: A pathogenesis-directed therapy. JAMA Dermatol. 2015;151:1110-12.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and tolerability of oral upadacitinib monotherapy in patients with recalcitrant vitiligo. J Am Acad Dermatol. 2023;89:1257-59.
- [CrossRef] [PubMed] [Google Scholar]
- Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-55.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous JAK expression in vitiligo. J Cutan Med Surg. 2021;25:157-62.
- [CrossRef] [PubMed] [Google Scholar]
- Vitiligo: Pathogenesis and new and emerging treatments. Int J Mol Sci. 2023;24
- [CrossRef] [Google Scholar]





