Translate this page into:
Role of C4d immunohistochemical marker in the diagnosis of bullous pemphigoid: A cross-sectional study
Corresponding author: Dr. Vijaya Basavaraj, Department of Pathology, Jagadguru Sri Shivarathreeshwara Medical College, JSS Academy of Higher Education and Research, Mysuru, India. vijayabasavaraj@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sneha Muppala R, Basavaraj V. Role of C4d immunohistochemical marker in the diagnosis of bullous pemphigoid: A cross-sectional study. Indian J Dermatol Venereol Leprol. 2024;90:569-74. doi: 10.25259/IJDVL_124_2023
Abstract
Objective
To determine the diagnostic utility of C4d immunohistochemical marker in cases of bullous pemphigoid by calculating the sensitivity, specificity, positive predictive value and negative predictive value.
Methods
We conducted an exploratory study (retrospectively and prospectively) from January 2017 to June 2022. All direct immunofluorescence proven cases of bullous pemphigoid were included in the study while cases with inadequate tissue for immunohistochemistry studies were excluded.
Results
Among the 57 cases of bullous pemphigoid, 49 showed positivity for C4d marker. All the ten control cases of inflammatory dermatoses were negative for C4d staining. A sensitivity of 86%, a specificity of 100%, a positive predictive value of 100% and a negative predictive value of 55.56% were calculated with a confidence interval of 95%.
Limitation
It is a single centre study. Selection bias may come into play.
Conclusion
Direct immunofluorescence on fresh or frozen skin tissue remains the gold standard. But in circumstances where direct immunofluorescence facilities are not available, C4d immunohistochemistry marker staining on formalin-fixed paraffin-embedded material submitted for standard microscopic investigation can, in most cases, confirm the diagnosis of bullous pemphigoid, obviating the need for a second biopsy.
Keywords
Bullous pemphigoid (BP)
direct immunofluorescence (DIF)
C4d
immunohistochemical marker (IHC)
Introduction
Among the autoimmune blistering diseases, bullous pemphigoid (BP) is the commonest, often affecting the elderly population. Drug-induced episodes, increased life expectancy and advancements in the detection of non-bullous presentation of the disease have all been linked to recent increases in incidence rates. It is characterised by blisters that are usually tense and overlying urticarial or erythematous plaques originating from the trunk and extending to the extremities, along with severe itching. The basement lining membrane of the dermo-epidermal junction (DEJ) is degraded as a result of dysregulated immunological T-cell response, neutrophil chemotaxis and the production of autoantibodies, primarily of IgG and IgE types against the hemidesmosomal bullous pemphigoid antigens (BP180 and BP230) leading to the development of subepidermal bullae. Involvement of the mucosa is rarely described.
The diagnosis is based on histological analysis showing subepidermal bulla with eosinophils or eosinophilic spongiosis and the identification of C3 and/or IgG deposits along the dermo-epidermal junction using direct immunofluorescence (DIF) testing or indirect immunofluorescence (IIF) testing, along with ELISA for measuring circulating BP180 and/or BP230 autoantibodies.
Gold standard in diagnosing bullous pemphigoid is DIF. However, the necessity for frozen or fresh tissue that is to be submitted in Michel’s solution or 0.9% NaCl, the requirement of laboratory equipment for performing the procedure, the possible loss of reactive antigens after storing the frozen tissue and the relatively high expenses are all limitations of this procedure.
The role of complement pathways in the pathogenesis of bullous pemphigoid is well established. Utilising immunoenzymatic techniques, commercially available rabbit and mouse polyclonal and monoclonal antibodies capable of reliably detecting human C3d and C4d which are stable components of classical complement pathway activation on formalin-fixed paraffin-embedded biopsies have been developed. The C4d immunohistochemistry (IHC) marker is used for detecting antibody-mediated rejection in transplanted kidneys. We analysed the expression of C4d in bullous pemphigoid as a possible substitute for DIF, as it is more stable and persistent in formalin-fixed paraffin-embedded tissue.
Materials and Methods
We conducted an exploratory study (retrospectively and prospectively) from January 2017 to June 2022. All DIF proven cases of bullous pemphigoid were eligible for inclusion in the study while cases with inadequate tissue for IHC studies were excluded. The formalin-fixed paraffin-embedded perilesional skin biopsies of the 57 DIF proven bullous pemphigoid cases were subjected to C4d IHC staining along with the 10 cases of inflammatory dermatoses. Antibody-mediated kidney transplant rejection biopsy was used as a positive control. The diagnostic ability of C4d immunohistochemical markers was assessed by calculating the sensitivity, specificity and predictive values.
Results
Fifty-seven cases of bullous pemphigoid received in the institution that were both clinico-histopathologically and DIF proven were considered for this study.
Age and gender distribution
Most patients fell under the age bracket of 51–90 years, having a mean age of 67.71 years with the age of patients ranging from 17 years to 102 years. Out of a total of 57 cases, 32 (56.1%) were females and 25 (43.9%) were males with a F:M ratio of 1.28.
Clinical presentation
Most of the patients (91.2%) presented with vesiculobullous lesions over urticarial or erythematous plaques on the trunk and/or extremities or as a generalised spread over the body. Mucosal involvement was seen in only 5 out of 57 cases (8.8%).
Site of biopsy
The majority of cases had biopsies taken from lesions over the trunk, arm, forearm and thigh. In a few cases, biopsies were taken from lesions over the leg, foot, wrist and knee.
Histopathology
Bullous pemphigoid is a subepidermal autoimmune blistering disease characterised by eosinophilic spongiosis and/or subepidermal bulla. The majority of cases showed subepidermal bullae (84.2%), followed by intraepidermal bullae (5.3%) and subepidermal bullae with focal intraepidermal location (3.5%). The presence of intraepidermal bullae may be attributed to regeneration of the epidermis at the floor of the split. Most of the cases showed inflammation composed of lymphocytes and eosinophils with a few also including neutrophils and mast cells.
Direct immunofluorescence
Out of 57 cases, 55 showed deposits of IgG and C3 along the dermo-epidermal junction with one case each showing only C3 deposits and only IgG deposits along the dermo-epidermal junction.
Immunohistochemistry expression of C4d
Expression of C4d in the DIF proven bullous pemphigoid cases showed positivity in 49 cases, seen as linear staining along the dermo-epidermal junction and encircling basal keratinocytes in 20 cases [Figure 1]; linear staining along the dermo-epidermal junction in 16 cases [Figure 2]; encircling basal keratinocytes in 9 cases [Figure 3]; discontinuous linear staining along the dermo-epidermal junction in 3 cases [Figure 4]; and focally encircling basal keratinocytes in 1 case [Figure 5]. The remaining 8 cases were negative for the C4d marker.
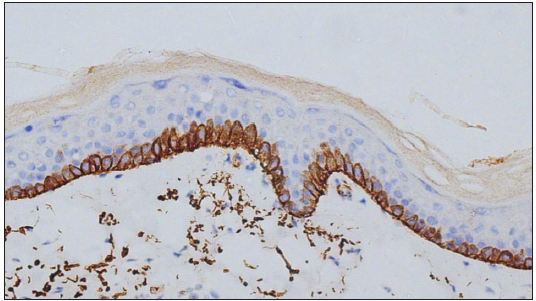
- Linear deposits of C4d IHC marker along the dermo-epidermal junction and encircling basal keratinocytes (400×).
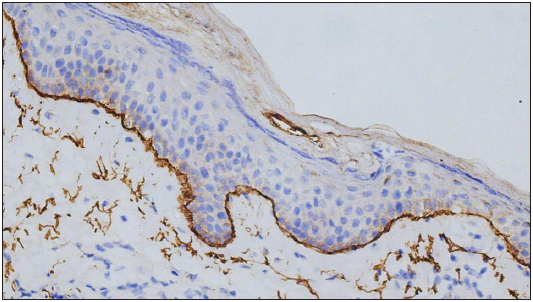
- Linear deposits of C4d IHC marker along the dermo-epidermal junction (400×).
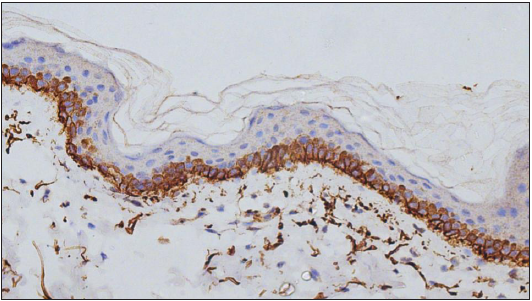
- Deposits of C4d IHC marker encircling basal keratinocytes (400×).
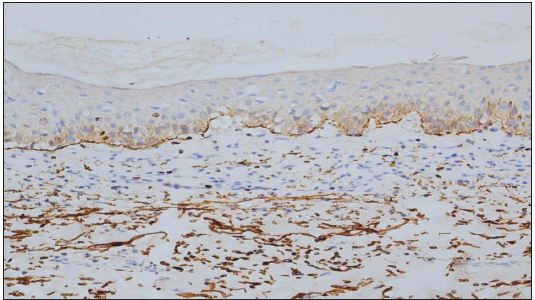
- Discontinuous linear deposits of C4d IHC marker (200×).
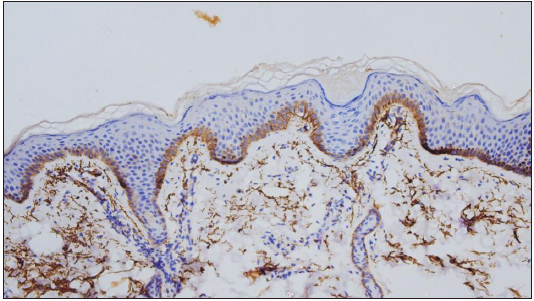
- Focal deposits of C4d IHC marker encircling basal keratinocytes (200×).
All 10 cases of inflammatory dermatoses (psoriasis: 4, psoriasiform dermatitis: 4, lichenoid dermatitis: 1 and non-specific dermatitis: 1) that were taken for assessing the specificity of the C4d marker were negative.
Calculation of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV)
Out of the 57 bullous pemphigoid cases, 49 were positive for C4d which constitutes the true positive cases. The remaining eight cases were negative for C4d which constitutes the false negative cases. Out of the ten inflammatory dermatoses control cases, all were negative for C4d which constitutes the true negative cases with a false positive of zero cases.
Sensitivity = TP/TP + FN = 49/(49 + 8) = 86%
Specificity = TN/TN + FP = 10/(10+0) = 100%
Positive predictive value = TP/TP+FP = 49/(49 + 0) = 100%
Negative predictive value = TN/TN + FN = 10/(10 + 8) = 55.56%
Using the above formulae, a sensitivity of 86%, a specificity of 100%, a positive predictive value of 100% and a negative predictive value of 55.56% were calculated.
Site of biopsy and C4d staining
C4d staining showed positivity in most of the biopsies taken from the trunk (21), arm (13), forearm (13) and thigh (6). However, all biopsies from the leg (1), foot (1), wrist (1) and knee (1) were negative for C4d.
In order to assess the possibility of an association between the site of the biopsy and the C4d IHC staining, we performed a chi-square test. P-value of 0.069 was obtained, indicating that there was no statistically significant association [Table 1].
| Site of biopsy | C4d Positive (%) | C4d negative (%) | Total (%) |
|---|---|---|---|
| Trunk & upper extremity | 43 (87.8) | 5 (62.5) | 48 (84.2) |
| Lower extremity | 6 (12.2) | 3 (37.5) | 9 (15.8) |
| Total | 49 (100) | 8 (100) | 57 (100) |
| Chi-square test | p-value | 0.069 |
Discussion
In 1953, Lever first coined the word ‘pemphigoid’ to distinguish a condition that was characterised by blisters formed as a result of detachment of the sub epidermis from ‘pemphigus’ which is a disorder characterised by blisters formed intraepidermally, due to acantholysis. The main methods for diagnosing bullous pemphigoid include clinical assessment, histopathological analysis and DIF studies.
The present study had patients in the age range of 17 years–102 years. Majority of them fell in the age bracket of 51–90 years with a mean age of 67.71 years. In various cohorts around the world, mean age has been shown to vary from 66 to 83 years.1 The gender distribution showed a female preponderance with an F:M ratio of 1.28. In the majority of the research, there was a clear female preponderance with an F:M ratio varying from 1.04 to 5.1.1–5 According to several studies, women appear to have a higher incidence rate up to the age of 75, but after that, men have a higher incidence rate.5–7
The majority of cases presented with vesiculobullous lesions over urticarial or erythematous plaques on the trunk and/or extremities or as a generalised spread over the body. Mucosal involvement was seen in 8.8% of the cases. A recent study by Kridin et al. found mucosal involvement in 17.1% of patients with bullous pemphigoid which was associated with disease severity.8
The skin biopsies from the perilesional area showed skin displaying subepidermal bullae containing fibrin and mixed inflammatory infiltrate predominantly composed of eosinophils and lymphocytes in the majority of cases with some also displaying intraepidermal bullae which can be attributed to regeneration of the epithelium at the floor of the bulla. A few cases also showed occasional neutrophil and mast cell inflammation. The cases of bullous pemphigoid that showed no bullae on histopathologic examination had features of eosinophilic spongiosis. This is in accordance with other studies on the histopathological examination of bullous pemphigoid.9,10
Gold standard in diagnosing bullous pemphigoid is DIF, where deposits of C3 and IgG can be seen along the dermo-epidermal junction.11 The DIF findings in our study showed that 55 out of 57 cases (96.5%) had both IgG and C3 deposits with one case having only IgG deposits and another having only C3 deposits. Despite being the gold standard, the necessity for frozen or fresh tissue that is to be submitted in Michel’s solution or 0.9% NaCl, the requirement of laboratory equipment for performing the procedure, the possible loss of reactive antigens after storing the frozen tissue and the relatively high expenses are all limitations of this procedure.
Usually, only tissue fixed in formalin is sent for HPE in centres where DIF facilities are not available. The efficacy of IHC done on formalin-fixed paraffin-embedded skin biopsies to demonstrate Ig and complement deposition is yet to be established. Also, the sensitivity of DIF on formalin-fixed paraffin-embedded tissue has been shown to be very low when compared to that done on fresh or frozen skin tissue biopsy.12 In recent studies, IHC deposition of C4d and C3d in formalin-fixed paraffin-embedded skin biopsies of inflammatory disorders was described as a useful diagnostic method.13,14 We analysed the expression of C4d in bullous pemphigoid as a possible substitute for DIF, as it is more stable and persistent in formalin-fixed paraffin-embedded tissue.15 In biopsies subjected to C4d where bullae were present, C4d deposits were seen along the roof and floor of the split. The immuno reactants were also seen around the hair follicle epithelium and also around eccrine glands along the basement membrane zone. Other studies on C4d deposition conducted by Villani et al. and Kamyab et al. also revealed similar patterns.16,17 In addition, Kamyab et al. also observed civatte-like deposition of C4d [Table 2].
A sensitivity of 86%, specificity of 100%, positive predictive value of 100% and negative predictive value of 55.56% was calculated which was compared and found to be in concordance with other studies done to evaluate C4d IHC staining in bullous pemphigoid16–19 [Table 3]. The calculated negative predictive value in our study was lower due to the relatively smaller number of control cases.
| Author of study | Pattern of C4d expression in bullous pemphigoid (no. of cases) | No. of cases positive for C4d |
|---|---|---|
| Villani et al.16 |
- Linear deposits along the dermo-epidermal junction (DEJ) - Encircling basal keratinocytes |
25/29 (86.2%) |
| Kamyab et al.17 |
- Linear deposits along the dermo-epidermal junction (18) - Encircling basal keratinocytes (8) - Civatte-like (3) |
27/30 (90%) |
| Present study |
- Linear deposits along the dermo-epidermal junction and encircling basal keratinocytes (20) - Linear deposits along the dermo-epidermal junction (16) - Encircling basal keratinocytes (9) - Discontinuous linear staining (3) - Focally encircling basal keratinocytes (1) |
49/57 (86%) |
| Parameters | Chandler W et al.18 | Kwon et al.19 | Villani et al.16 | Kamyab et al.17 | Present study |
|---|---|---|---|---|---|
| Year | 2009 | 2013 | 2015 | 2019 | 2022 |
| Country | USA | USA | France | Iran | India |
| Sensitivity | 77.7% | 83.3% | 86.2% | 90% | 86% |
| Specificity | 100% | 100% | 98% | 86.7% | 100% |
| Positive predictive value | 100% | 100% | 96% | 87% | 100% |
| Negative predictive value | 66.7% | 92% | 92% | 89.7% | 55.56% |
In order to assess the possibility of an association between the site of the biopsy and the C4d IHC staining, we performed a chi-square test. P-value of 0.069 was obtained, indicating that any difference in findings based on the site of biopsy can be attributed to chance.
The complement system (CS) plays an important role in innate immunity. It serves as a defence mechanism against infectious agents and maintains tissue homoeostasis. The complement system can be triggered by three main pathways: the classical pathway (CP), lectin pathway (LP) and alternative pathway (AP). The classical pathway is activated through the binding of C1q. The activation of lectin pathway occurs through the binding of mannose-binding lectin (MBL) and the alternative pathway initiates spontaneously, but factors like properdin and factor B (FB) may act as recognition molecules.20
In 1968, the binding of complement to the basement membrane in cases of bullous pemphigoid was first demonstrated in vivo by Chorzelski and Cormane.21 Further research conducted by Jordon and his colleagues revealed that bullous pemphigoid autoantibodies fix not only C3, C1q and C4 but also factor B and properdin, suggesting the involvement of both classical and alternative pathway activation. In addition, C3 deposits were also found in cases where no autoantibodies were detected at the dermo-epidermal junction.22 Liu et al. conducted experiments on BALB/c neonatal mice by introducing rabbit polyclonal autoantibodies against the NC14A domain which is a homologue of the NC16A domain in humans. No subepidermal blistering occurred when they depleted serum complement with cobra venom factor, induced a state of C5 deficiency and used Fab fragments of anti-murine autobodies against BP180.23 Another study by Nelson et al. showed protection from the development of bullous pemphigoid when C4-deficient mice were treated with anti-C1q antibodies. This highlights the role of the classic complement pathway in the development of bullous pemphigoid.24 The study also demonstrated less intense and delayed blister formation in factor B–deficient mice which highlights the minor involvement of alternative complement pathway as well. They also conducted studies on humanised mouse models which had the NC16A domain and got similar results.25 C5 has also been implicated in the pathogenesis of bullous pemphigoid. In a study by Karsten et al., 50% of the mice with knockout of C5 showed reduced disease activity. It also demonstrated the differing roles of C5a receptors, i.e., C5aR1 and C5aR2. Induced C5aR1 deficiency had a protective effect, while C5aR2 deficiency had a pro-inflammatory effect. When given treatment with the C5aR1 inhibitor PMX53 peptide before the development of the disease, it was shown to halt disease progression.26 This emphasises the role of the complement system during the early stages of the disease. When C5a interacts with C5aR1, mast cell degranulation takes place, releasing proinflammatory cytokines that attract neutrophils, eosinophils, proteases and MMP-9. Neutrophil protease and MMP-9 cleave BP180 which leads to the development of subepidermal blisters.27 C3a and C5a can directly degranulate eosinophils as well which also leads to blister formation.28
All of these findings indicate that the infiltration of specific effector cells and their activation which are responsible for the characteristic subepidermal blisters in bullous pemphigoid are influenced, at least indirectly, by an activated complement cascade. On this note, recently, studies have been done to assess the diagnostic capability of C3d and C4d IHC markers on formalin-fixed paraffin-embedded tissue in cases of bullous pemphigoid. C3d was shown to have a higher sensitivity, ranging from 97 to 100%,29,30 while C4d had a sensitivity ranging from 78 to 90%.16–19 C4d IHC marker is used for detecting antibody-mediated rejection in transplanted kidneys. In our study, we selected the C4d IHC marker as it is more stable and persists in formalin-fixed paraffin-embedded tissue. These findings suggest that a combination of C3d and C4d IHC markers, when feasible, or IHC staining with either C3d or C4d markers are valuable diagnostic tools in cases of bullous pemphigoid, especially when a DIF facility is not available.
Limitation of the study: Selection bias may come into play as it is a single centre study. The characteristics of the patients from a single centre may differ from those at other centres which can limit the external validity of the study. In addition, there may be limited diversity in terms of demographic, socioeconomic and clinical characteristics of the participants. The study may also be influenced by the referral patterns of the institution.
Conclusion
In circumstances where DIF facilities are not available, C4d IHC marker staining on formalin-fixed paraffin-embedded material submitted for standard microscopic investigation can, in most cases, confirm the diagnosis of bullous pemphigoid, obviating the need for a second biopsy. This method can also be utilised for research because formalin-fixed paraffin-embedded material remains available for a number of years.
Acknowledgement
The authors immensely appreciate the support of the staff of the Department of Dermatology for providing them the cases and also thank the expertise of the technical staff in the hospital laboratory.
Ethical approval
The research was approved by the Institutional Ethics Committee at JSS Medical College & Hospital, Mysuru, number JSS/MC/PG/5156/2020-21, dated 29th January 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Pemphigus in the mediterranean region of Turkey: A study of 148 cases. Int J Dermatol. 2006;45:523-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris is the most common autoimmune bullous disease in northwestern Romania. Int J Dermatol. 2010;49:768-74.
- [CrossRef] [PubMed] [Google Scholar]
- Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: Population based cohort study. BMJ. 2008;337
- [Google Scholar]
- Spectrum of autoimmune bullous diseases in Kuwait. Int J Dermatol. 2004;43:876-81.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132:1998-2004.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2‐year prospective study. Br J Dermatol. 2009;161:861-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ethnic variations in the epidemiology of bullous pemphigoid in Israel. Int J Dermatol. 2018;57:34-9.
- [Google Scholar]
- Assessment of the prevalence of mucosal involvement in bullous pemphigoid. JAMA Dermatol. 2019;155:166-71.
- [Google Scholar]
- Eosinophilic spongiosis in bullous pemphigoid. Arch Dermatol. 1984;120:1166-8.
- [PubMed] [Google Scholar]
- Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-53.
- [CrossRef] [PubMed] [Google Scholar]
- The value of direct immunofluorescence on proteinase-digested formalin-fixed paraffin-embedded skin biopsies. Am J Dermatopathol. 2018;40:111-7.
- [CrossRef] [PubMed] [Google Scholar]
- The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59:822-33.
- [CrossRef] [PubMed] [Google Scholar]
- Complement in bullous pemphigoid: Results from a large observational study. Br J Dermatol. 2017;176:517-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bullous pemphigoid: use of C4d immunofluorescent staining in a case with repeated negative conventional direct immunofluorescence studies. Am J Dermatopathol. 2017;39:932-4.
- [CrossRef] [PubMed] [Google Scholar]
- Application of C4d immunohistochemistry on routinely processed tissue sections for the diagnosis of autoimmune bullous dermatoses. Am J Dermatopathol. 2016;38:186-8.
- [CrossRef] [PubMed] [Google Scholar]
- C4d immunohistochemical stain of formalin‐fixed paraffin‐embedded tissue as a sensitive method in the diagnosis of bullous pemphigoid. J Cutan Pathol. 2019;46:723-8.
- [CrossRef] [PubMed] [Google Scholar]
- C4d immunohistochemical stain is a sensitive method to confirm immunoreactant deposition in formalin‐fixed paraffin‐embedded tissue in bullous pemphigoid. J Cutan Pathol. 2009;36:655-9.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of C4d immunohistochemistry on formalin-fixed paraffin-embedded tissue in the distinction of polymorphic eruption of pregnancy from pemphigoid gestationis. Am J Dermatopathol. 2013;35:787-91.
- [CrossRef] [PubMed] [Google Scholar]
- The relevance of complement in pemphigoid diseases: A critical appraisal. Front Immunol. 2022;13
- [Google Scholar]
- The presence of complement “bound” in vivo in the skin of patients with pemphigoid. Dermatologica. 1968;137:134-8.
- [CrossRef] [PubMed] [Google Scholar]
- The complement system in bullous pemphigoid: VI. C3 fixing activity in the absence of detectable antibody. Clin Immunol Immunopathol. 1979;13:475-83.
- [CrossRef] [PubMed] [Google Scholar]
- The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95:1539-44.
- [CrossRef] [PubMed] [Google Scholar]
- Role of different pathways of the complement cascade in experimental bullous pemphigoid. J Clin Invest. 2006;116:2892-900.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5aR2. Front Immunol. 2018;9:488.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The C5a receptor on mast cells is critical for the autoimmune skin-blistering disease bullous pemphigoid. J Biol Chem. 2011;286:15003-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Degranulation from human eosinophils stimulated with C3a and C5a. Int Arch Allergy Immunol. 1994;104:27-9.
- [Google Scholar]
- C3d immunohistochemistry on formalin‐fixed tissue is a valuable tool in the diagnosis of bullous pemphigoid of the skin. J Cutan Pathol. 2010;37:654-8.
- [CrossRef] [PubMed] [Google Scholar]
- The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59:822-33.
- [CrossRef] [PubMed] [Google Scholar]






