Translate this page into:
Role of dental restoration materials in oral mucosal lichenoid lesions
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
3 Department of Oral Health Sciences, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Sanjeev Handa
Professor and Head, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012
India
| How to cite this article: Sharma R, Handa S, De D, Radotra BD, Rattan V. Role of dental restoration materials in oral mucosal lichenoid lesions. Indian J Dermatol Venereol Leprol 2015;81:478-484 |
Abstract
Background: Dental restorative materials containing silver-mercury compounds have been known to induce oral lichenoid lesions. Objectives: To determine the frequency of contact allergy to dental restoration materials in patients with oral lichenoid lesions and to study the effect of removal of the materials on the lesions. Results: Forty-five patients were recruited in three groups of 15 each: Group A (lesions in close contact with dental materials), Group B (lesions extending 1 cm beyond the area of contact) and Group C (no topographic relationship). Thirty controls were recruited in two groups of 15 individuals each: Group D (oral lichenoid lesions but no dental material) and Group E (dental material but no oral lichenoid lesions). Patch tests were positive in 20 (44.5%) patients. Mercury was the most common allergen to elicit a positive reaction in eight patients, followed by nickel (7), palladium (5), potassium dichromate (3), balsam of Peru, gold sodium thiosulphate 2 and tinuvin (2) and eugenol (1), cobalt chloride (1) and carvone (1). Seven patients elicited positive response to more than one allergen. In 13 of 20 patients who consented to removal of the dental material, complete healing was observed in 6 (30%), marked improvement in 7 (35%) and no improvement in 7 (35%) patients. Relief of symptoms was usually observed 3 months after removal. Limitations: Limited number of study subjects and short follow up after removal/replacement of dental restoration materials are the main limitations of this study. Conclusion: Contact allergy to amalgam is an important etiologic factor in oral lichenoid lesions and removal of restorative material should be offered to patients who have lesions in close proximity to the dental material.INTRODUCTION
Oral lichen planus is a relatively common mucocutaneous disease affecting about 0.1-4% of the population. [1],[2] Finne in 1982 proposed the term oral lichenoid reactions to designate lesions that were clinically indistinguishable from oral lichen planus but in which a specific etiological factor could be inferred and/or demonstrated. [3] Oral lichenoid lesions comprise of both oral lichen planus and oral lichenoid reaction. Silver-mercury amalgam has been used as a dental restorative material since the early 19 th century. It is strong, long lasting, well fitting, easy to handle and cheap. [4] Mercury and mercury compounds appear to be the most common allergens in amalgam-induced oral lichenoid lesions; other metals being rarely responsible for allergic reactions. This is particularly true in present times due to increasing trends in body piercing, tattooing and wearing of jewelry in both genders seen worldwide. It potentially enhances the likelihood of exposure to metallic and non-metallic materials which are also used in dental restorations and orthodontic appliances. [5],[6],[7] Oral lichenoid reaction to metals other than mercury and mercury compounds used in dentistry, such as cadmium chloride, cobalt chloride, potassium dichromate, gold sodium thiosulfate, palladium chloride, platinum and silver have been reported in various studies. [5],[8] We studied the frequency of positive patch test reactions to constituents of dental amalgam in patients with oral lichenoid lesions and controls. In addition, the effect of replacement of amalgam fillings on oral lichenoid lesions in patients with positive patch test(s) was also studied.
Materials and Methods
This prospective controlled study included 75 consecutive patients, 47 women and 28 men, attending the department of dermatology or oral health sciences centre of Postgraduate Institute of Medical Education and Research, Chandigarh between January 2012 and June 2013. All prospective patients had been screened for the presence of oral lichenoid lesions and in situ dental restoration material. Patients aged less than 18 years, those unwilling to undergo either patch testing with dental series of allergens or mucosal biopsy, and habitual tobacco and betel nut chewers were excluded from the study.
The diagnosis of oral lichenoid lesion was mainly clinical and confirmed by histopathologic examination. Histologically, features such as varying degree of focal hyperkeratosis or parakeratosis, irregular acanthosis or atrophy, liquefaction degeneration of the basal cell layer, a dense band-like lymphocytic infiltrate high in the lamina propria, and whenever observed, hyaline (Civatte) bodies, which represent degenerated basal cells, were considered confirmatory for oral lichen planus. Differentiation of oral lichenoid reaction from oral lichen planus was based on criteria proposed by Thornhill et al., [9] which include an inflammatory infiltrate deeply located in some or all areas, a focal perivascular infiltrate and the presence of plasma cells and neutrophils.
Oral lichenoid lesions were classified clinically into three types: (i) white patches, striated, plaque or reticular lesions, (ii) erosive/ulcerative lesions or atrophic lesions and (iii) papular and bullous lesions. Lesional location in all patients was categorized as oral lichenoid lesions located on the buccal mucosa (unilateral or bilateral), tongue (lateral surfaces or dorsal surface), gingivae and other parts of oral mucosa such as lips, floor of the mouth and palate.
Consecutive patients (eligible and consenting) were recruited into the individual groups till the required number of 15 was reached. In Group A, the oral lichenoid lesions were confined to areas in close contact with dental materials, Group B had lesions extending 1 cm beyond the area of contact with dental materials and in Group C, the oral lesions did not have any topographic relationship with the dental materials. Control subjects included two groups of 15 patients each: Group D patients had an oral lichenoid lesion and no dental restoration materials and Group E patients had dental restoration materials but no oral lichenoid lesion.
Patch tests to detect sensitivity to the constituents of dental materials were carried out by Finn chamber method using dental material series sourced from Chemotechnique Diagnostics, Sweden [Table - 1]. Readings of patch test were performed on Day 2 and 4 and graded using the International Contact Dermatitis Research Group criteria. Patients who were patch test positive to any allergen were advised to get dental material removed/replaced with other materials. Those who complied with this advice were further examined for clinical remission at 1, 3 and 6 months. Clinical responses were graded as complete healing (absence of clinical signs and symptoms), marked improvement (i.e., decrease in size and/or a less severe clinical form of the lesion) and no improvement or worse.

The results were analyzed statistically by means of exact Chi-square test. All statistical results were performed in which two-sided P values were calculated (with statistical significance set at P < 0.05).
RESULTS
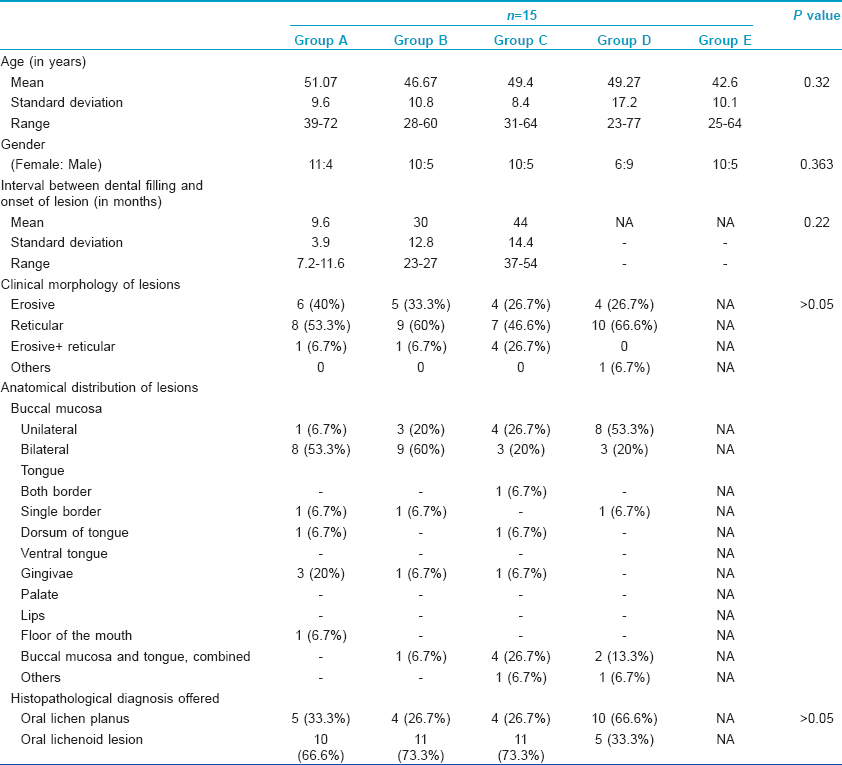
The baseline demographic details, interval between dental filling and onset of lesions, morphology of lesions and their anatomical distribution in all the 75 study subjects including controls are summarized in [Table - 2]. According to the histopathological criteria used, there were 37 (61.7%) study subjects with oral lichenoid lesion/oral lichenoid reaction and 23 (38.3%) subjects with oral lichen planus [Table - 2].
Patch test result
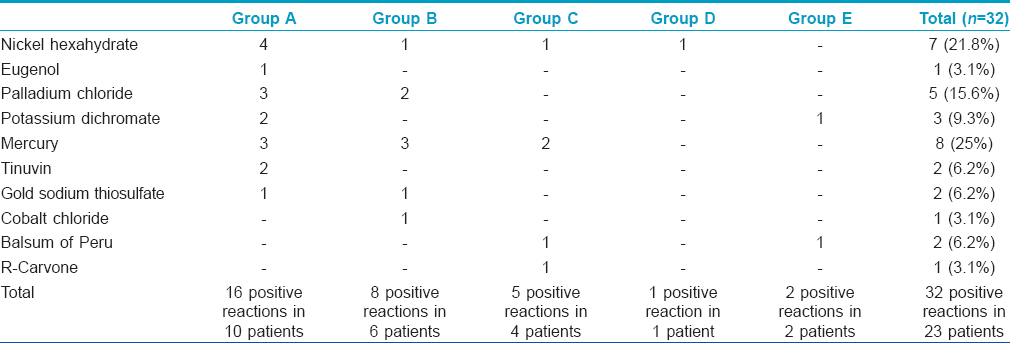
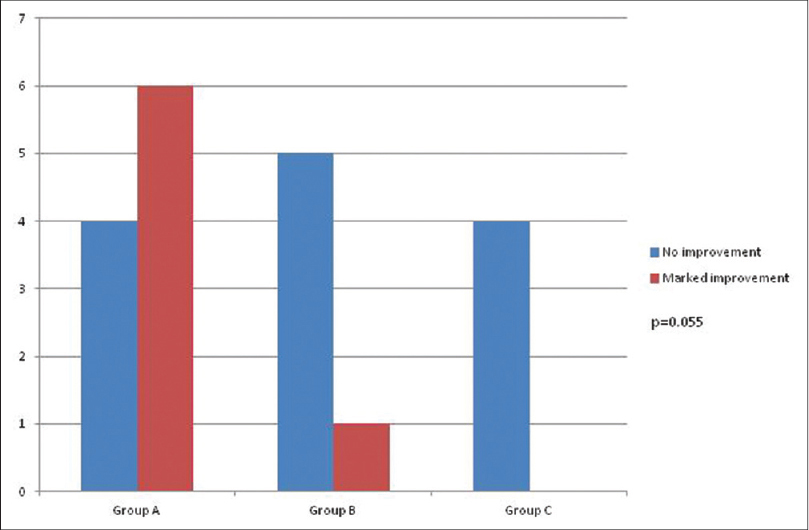
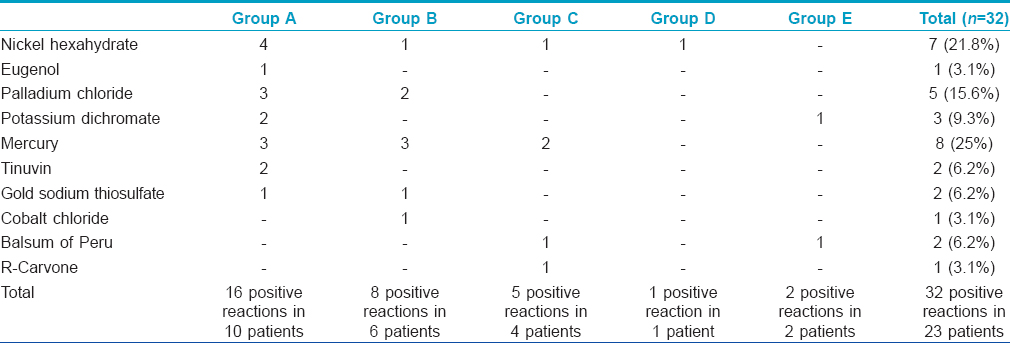
Positive patch tests in either of two readings were observed to 29 allergens in 20 (44.5%) patients across three groups and to three allergens in three (10%) subjects from both the control groups [Table - 3], [Figure - 1] and [Figure - 2]. Thus, overall there were 32 positive patch test reactions in 23 subjects. Eight (25%) positive reactions were due to mercury compounds followed by 7 (21.8%) with nickel and 5 (15.6%) with palladium. Ten (66.7%) patients in Group A had a positive patch test reaction to at least one allergen eliciting a total of 16 positive patch test reactions, while the corresponding figures were 6 (40%) patients in Group B with 8 positive reactions, 4 (26.7%) patients in Group C with 5 positive reactions, 1 (6.7%) subject in Group D and 2 (13.4%) subjects in Group E, respectively. None of them showed positive reactions to resin or acrylate dental materials such as methyl methacrylate, bisphenol A, ethylene glycol dimethacrylate and triethyleneglycol dimethacrylate.
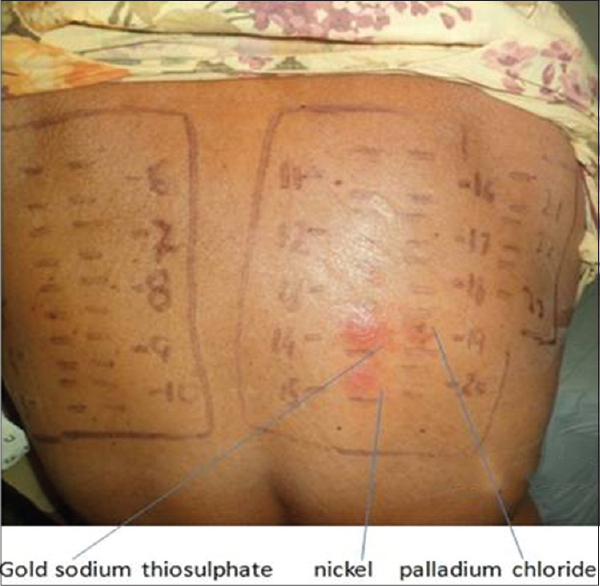 |
| Figure 1: Positive patch test reaction (2+) to gold sodium thiosulphate, (2+) to nickel and (1+) to palladium chloride |
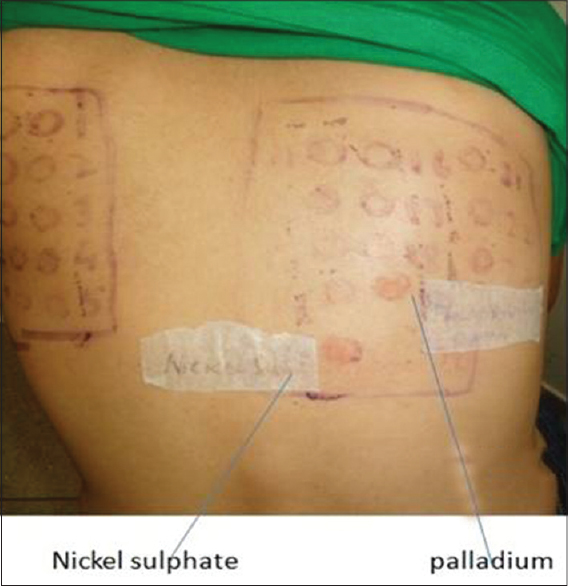 |
| Figure 2: Positive patch test reaction (1+) to nickel sulfate and palladium chloride |
Healing of oral lichenoid lesions
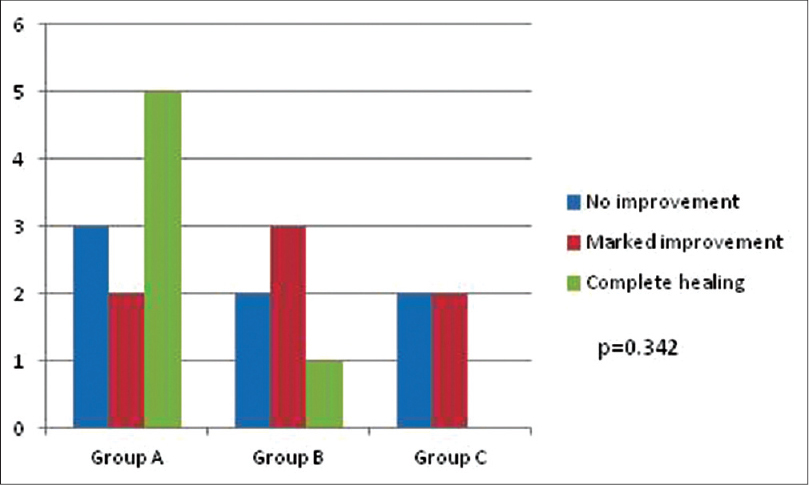
Removal/replacement of dental restoration material was offered to patients who reacted to at least one allergen. The healing of lesions according to anatomical area of involvement in different groups is summarized in [Table - 4]. No improvement was noted at one month after replacement/removal of amalgam in any of the patients. Three months after replacement/removal of amalgam, marked improvement was noticed in seven (35%) patients; six in Group A (oral lichenoid lesion in close proximity to dental restoration), and one in Group B (oral lichenoid lesion extending 1 cm beyond contact with dental restoration) [Figure - 3], [Figure - 4], [Figure - 5]. The probability of marked improvement in lesions almost reached statistical significance (P = 0.055) with significantly more numbers in Group A having marked improvement. Lesions on buccal mucosa and tongue healed better than those involving the gingivae [Table - 4].
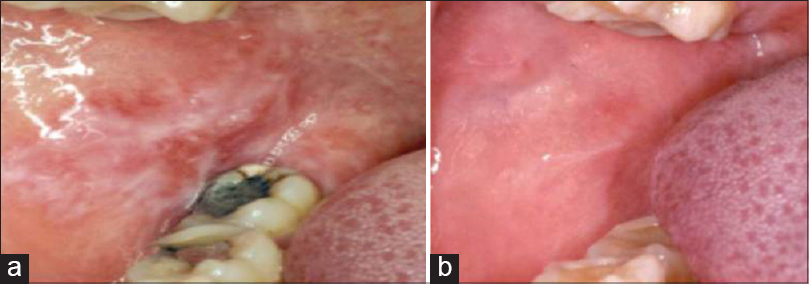 |
| Figure 3: (a) Oral lichenoid lesion adjacent to amalgam and extending beyond it. (b) Complete healing after 6 months of replacing amalgam restorations |
 |
| Figure 4: Improvement in oral lichenoid reaction, 3 months after removal/replacement of dental filling |
 |
| Figure 5: Improvement in oral lichenoid reaction, 6 months after removal/replacement of dental filling |
At 6 months post replacement/removal, complete healing was observed in six (30%), marked improvement in seven (35%) and no improvement in seven (35%) patients, respectively [Figure - 5]. In Group A (oral lichenoid lesion in close proximity to dental restoration), 5 (50%) of 10 patients had complete healing, whereas 2 (20%) patients had marked improvement. In Group B (oral lichenoid lesion extending beyond contact with dental restoration), one (16.6%) of the six patients showed complete healing while marked improvement was observed in three (50%) patients. Two (50%) of four patients in Group C (no topographic relation) had marked improvement. The difference in the healing of lesions 6 months after replacement/removal of dental restoration material in different groups was not statistically significant (P = 0.342).
DISCUSSION
A variety of clinical forms of oral lichenoid lesions have been described and commonly include reticular lesions which are the most common, plaque, papular, atrophic and bullous types. Although morphologically oral lichenoid lesions resemble oral lichen planus, some researchers suggest that the clinical profile of oral lichenoid lesions related to amalgam fillings differs from the classic clinical manifestations of oral lichen planus. [10] Most patients in our study had distinct reticular lesions mainly presenting unilaterally on buccal mucosa in proximity to amalgam. Erosive lesions were also observed additionally in 10% of them. It may sometimes be difficult to differentiate between oral lichen planus and oral lichenoid lesion/oral lichenoid reaction based solely on histopathology but biopsy helps to exclude lesions of pemphigus vulgaris, bullous pemphigoid, leukoplakia or lupus erythematosus that may occasionally mimic oral lichenoid lesion/oral lichen planus. Based on the histopathological criteria proposed by Thornhill et al., [9] we could classify 61.7% of our cases as oral lichenoid lesion/oral lichenoid reaction and 38.3% cases as oral lichen planus. The limitation of the criteria proposed by Thornhill et al., is it has not been validated in subsequent studies.
The incidence of contact sensitization from dental restoration materials varies between 13% and 60%. [7],[8],[11] The results of patch tests tend to vary substantially across studies depending upon components of dental restoration materials used or their concentration, vehicles used in the test and test evaluation criteria. [12] While mercury or its compounds are the most common allergens in most studies, gold sodium thiosulphate and palladium chloride were the most frequent allergens in a study by Raap et al. [8] Patch tests were positive to at least one allergen in 44.5% of our subjects across all groups; 66.7% in Group A, 40% in Group B, 26.7% in Group C, 6.7% in Group D and 13.4% in Group E with a total of 32 positive reactions. The most common allergen in dental amalgam eliciting positive patch test reaction was mercury in 25% followed by nickel (22%) and palladium (15.6%). Oral lichenoid lesions related to non-metallic dental materials have been relatively infrequent. All our patients showed negative reactions to resin and methacrylate dental materials.
It is well known that dental restoration materials may elicit positive patch test reactions in individuals who do not have clinically apparent oral lichenoid lesion or dental amalgam in situ. This occurs because of sensitization to allergens in dental series which are ubiquitously present otherwise. Positive patch tests observed in patients of our two control groups [presence of oral lichenoid lesion in absence of dental amalgam (Group D), and dental amalgam in absence of oral lichenoid lesion (Group E)] may have been sensitized similarly.
Occurrence of oral lichenoid lesions associated with dental amalgam is a well known phenomenon. Contact sensitivity has been observed to the constituents of the dental filling material in a significant proportion of such patients. Similarly, improvement/healing of lesions in patients who have an oral lichenoid lesion in close proximity to the dental amalgam irrespective of the results of patch test to constituents of dental amalgam, also suggests some etiological relationship. However, the disease course after replacement of amalgam is not uniform across the reported studies, thus, whether contact sensitivity is causal remains doubtful. Little et al. [13] also tried to determine whether mercury released from amalgam can directly act on keratinocytes to induce oral lichenoid lesions. They observed that subcytotoxic concentrations of mercury salt induced a concentration-dependent rise in intercellular adhesion molecule-1 expression and subsequent T-cell binding but the phenomenon occurred exclusively on oral mucosal keratinocytes. Additionally, it also stimulated the release of low levels of tumor necrosis factor-α and interleukin-8 while inhibiting the release of interleukin-1α by oral mucosal keratinocytes. [13] According to Wong and Freeman, oral lichenoid lesions may develop due to the irritant effect of mercury rather than being an allergic response as patch test negative patients too improved after amalgam replacement. [14] A possibility of local toxic effects of amalgam and the significance of a close topographical relationship between artificial material and pathological manifestation on the mucosa, too has been suggested by some researchers. [15] However, the exact etiopathogenesis of such lesions largely remains conjectural.
Treatment of oral lichenoid lesions related to contact allergy to dental restoration materials consists of removal, replacement or recovering of fillings in direct physical contact with mucosal lesions when suspected of playing a causal role. [12],[15] Dunsche et al. described regression in lichenoid changes after amalgam substitution in 97% of their 134 patients independent of patch test results. [15] Similarly, Lind et al. studied 52 patients with oral lichen planus topographically related to amalgam restorations. [16] Replacement of filling was done in 18 and 16 of them experienced complete remission of the lesions within 1-12 months. Similar observations were also made by Laeijendecker et al. after amalgam fillings were replaced to monitor the effect of partial or complete replacement of amalgam fillings following a positive patch test reaction to ammoniated mercury, metallic mercury or amalgam. [17] Lesions, particularly those in close proximity to the amalgam, healed or improved markedly in 13 of our 20 patients who were positive to patch test and agreed to the removal/replacement of their restorations. Healing was better in lesions on buccal mucosa and tongue compared with those on gingivae, possibly because of the close and fixed proximity of restoration material to gingivae.
CONCLUSIONS
Oral lichenoid lesion/oral lichenoid reaction appears to be a significant problem associated with dental restoration material particularly with silver-mercury amalgam and their removal leads to complete/partial healing of these lesions in the majority of patients. Topographical relationship between oral lichenoid lesions and dental restoration material appears clinically relevant and their removal should be offered to patients with lesions in close proximity to their restorations. Positive patch test results with dental restoration materials also provides some clue to the pathogenesis of oral lichenoid lesion/oral lichenoid reaction; if not all, some of them perhaps are due to the allergenic/irritant potential of these dental restoration materials. Small number of cases in all groups and lack of long-term follow up are some of the limitations of this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol 1991;25:593-619.
[Google Scholar]
|
| 2. |
Montazem A, Buonocore PM. Lichen planus and the vulvovaginal-gingival syndrome. J Periodontol 2003;74:1385-93.
[Google Scholar]
|
| 3. |
Finne K, Göransson K, Winckler L. Oral lichen planus and contact allergy to mercury. Int J Oral Surg 1982;11:236-9.
[Google Scholar]
|
| 4. |
Pang BK, Freeman S. Oral lichenoid lesions caused by allergy to mercury in amalgam fillings. Contact Dermatitis 1995;33:423-7.
[Google Scholar]
|
| 5. |
Garner A. Contact dermatitis to metals. Dermatol Ther 2004;17:321-7.
[Google Scholar]
|
| 6. |
Vamnes JS, Morken T, Helland S, Gjerdet NR. Dental gold alloys and contact hypersensitivity. Contact Dermatitis 2000;42:128-33.
[Google Scholar]
|
| 7. |
Issa Y, Duxbury AJ, Macfarlane TV, Brunton PA. Oral lichenoid lesions related to dental restorative materials. Br Dental J 2005;198:361-6.
[Google Scholar]
|
| 8. |
Raap U, Stiesch M, Reh H, Kapp A, Werfel T. Investigation of contact allergy to dental metals in 206 patients. Contact Dermatitis 2009;60:339-43.
[Google Scholar]
|
| 9. |
Thornhill MH. Oral lichenoid lesions and amalgam fillings. Evid Based Dent 2006;7:74-5.
[Google Scholar]
|
| 10. |
Henriksson E, Mattsson U, Håkansson J. Healing of lichenoid reactions following removal of amalgam. A clinical follow-up. J Clin Periodontol 1995;22:287-94.
[Google Scholar]
|
| 11. |
Ditrichova D, Kapralova S, Tichy M, Ticha V, Dobesov J. Oral lichenoid lesions and allergy to dental material. Biomed 2007;151:333-9.
[Google Scholar]
|
| 12. |
Al-Hashimi I, Schifter M, Lockhart B, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid lesions: Diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(Suppl):S25.e1-12.
[Google Scholar]
|
| 13. |
Little MC, Watson RE, Pemberton MN, Griffiths CE, Thornhill MH. Activation of oral keratinocytes by mercuric chloride: Relevance to dental amalgam- induced oral lichenoid reactions. Br J Dermatol 2001;144:1024-32.
[Google Scholar]
|
| 14. |
Wong L, Freeman S. Oral lichenoid lesions (OLL) and mercury in amalgam filling. Contact Dermatitis 2003;48:74-9.
[Google Scholar]
|
| 15. |
Dunsche A, Kästel I, Terheyden H, Springer, Christophers E, Brasch J. Oral lichenoid reactions associated with amalgam: Improvement after amalgam removal. Br J Dermatol 2003;148:70-6.
[Google Scholar]
|
| 16. |
Lind PO, Hurlen B, Lyberg T, Aas E. Amalgam-related oral lichenoid reaction. Scand J Dent Res 1986;94:448-51.
[Google Scholar]
|
| 17. |
Laeijendecker R, Dekker SK, Burger PM, Mulder PGH, Van Joost T, Neumann MH. Oral lichen planus and allergy to dental amalgam restorations. Arch Dermatol 2004;140:1434-8.
[Google Scholar]
|
Fulltext Views
4,605
PDF downloads
2,032





