Translate this page into:
Scanning electron microscopy study of hair shaft changes related to hardness of water
2 Department of Family and Community Medicine, College of Medicine, King Faisal University, Hofuf, Saudi Arabia
Correspondence Address:
Feroze Kaliyadan
Department of Dermatology, College of Medicine, King Faisal University, Hofuf
Saudi Arabia
| How to cite this article: Alahmmed LM, Alibrahim EA, Alkhars AF, Almulhim MN, Ali SI, Kaliyadan F. Scanning electron microscopy study of hair shaft changes related to hardness of water. Indian J Dermatol Venereol Leprol 2017;83:740 |
Abstract
Introduction and Aims: Brittleness and breakage of hair is a common complaint in the geographical area of Saudi Arabia where we work. This area has a high level of hardness in normal tap water. We aimed to study and compare structural differences and relative deposition of calcium and magnesium salts on the hair shaft surface using scanning electron microscopy (SEM) between hair shaft samples from normal, healthy volunteers treated with hard and soft water.Methods: Hair samples obtained from 20 healthy volunteers were divided into two groups. One group was treated with hard water for 3 weeks and the second with soft water for the same duration. SEM was used to assess hair shaft surface damages and relative deposition of calcium and magnesium on the surface of the hair.
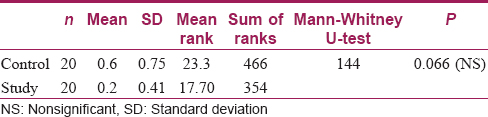
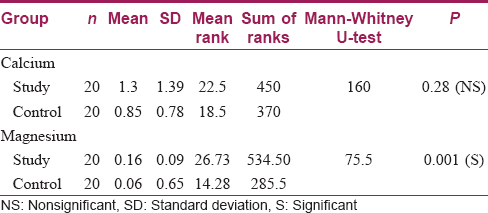
Results: There was no statistically significant difference between the study and control group as far as surface changes under SEM were concerned. As far as the relative deposition of calcium and magnesium was concerned, there was no statistically significant difference in calcium deposition between the control and study samples (P = 0.28). On the other hand, magnesium deposition showed a significant difference between both groups (P = 0.001), with a higher level in samples washed with hard water.
Conclusions: Hard water may be associated with increased deposits on the hair shaft surface, however, this does not necessarily translate into evident structural surface changes, as evidenced by SEM.
Introduction
Patients presenting with brittleness and breakage of the hair shaft are common in our outpatient department. Many of these patients attribute this to the hardness of water, which they use routinely for showering. Studies from this area have confirmed a high level of hardness in ground water.[1]
The “hardness” of water is directly related to the presence of salts like calcium carbonate and magnesium sulfate in the water. Bicarbonates contribute to temporary hardness, which can be removed by boiling, whereas the sulfates are primarily responsible for permanent hardness, which cannot be removed by boiling.[2],[3],[4] A study conducted by Srinivasan et al., based on hair from a sample of 15 healthy volunteers, concluded that there was no significant difference in the elasticity and tensile strength of hair treated with either hard water or soft water.[4] Another study by the same group used scanning electron microscopy (SEM) to study the surface of hair treated with hard and soft water.[5] The study concluded that hair treated with hard water has a higher mineral deposition, which, in turn, can lead to irregularity of the surface and decreased thickness of hair over a period of time. Our study focuses on the scanning electron microscopy of healthy hair to assess and compare surface changes after treatment with hard and soft water. The two parameters we used were structural changes in the hair shaft under SEM and the deposition of calcium and magnesium. We attempted to study the changes after simulating real-life usage.
Methods
Twenty healthy volunteers were selected after informed consent. All volunteers had no history of any significant skin or systemic diseases. Patients with a history of any significant cosmetic treatment (hair coloring/bleaching/perming/straightening) were excluded from the study. The samples were cut 5 cm from the root. Two hair samples of every single volunteer were obtained and separated into two different groups. In one group (the study group), hair samples were washed with hard water for 3 weeks. Normal tap water from our region was used in the study group because of its native hardness (average hardness – 287–533 ppm of CaCO3) and frequent washing (5 days of every week) was used to simulate real-life usage. Hair samples were exposed to hard water for a period of approximately 10 minutes every day (the days on which hairs were washed) for a total of 3 weeks. In the other group (control group) hair samples were washed with soft water (bottled drinking water – average hardness 50–250 ppm of CaCO3) using the same protocol used in the study group. The main parameters studied were
- Surface changes related to the hair shaft under SEM
- Relative deposition of calcium and magnesium on the hair samples.
The local Institutional Ethics Committee approved the study, and data confidentiality was assured.
Scanning electron microscopy procedure
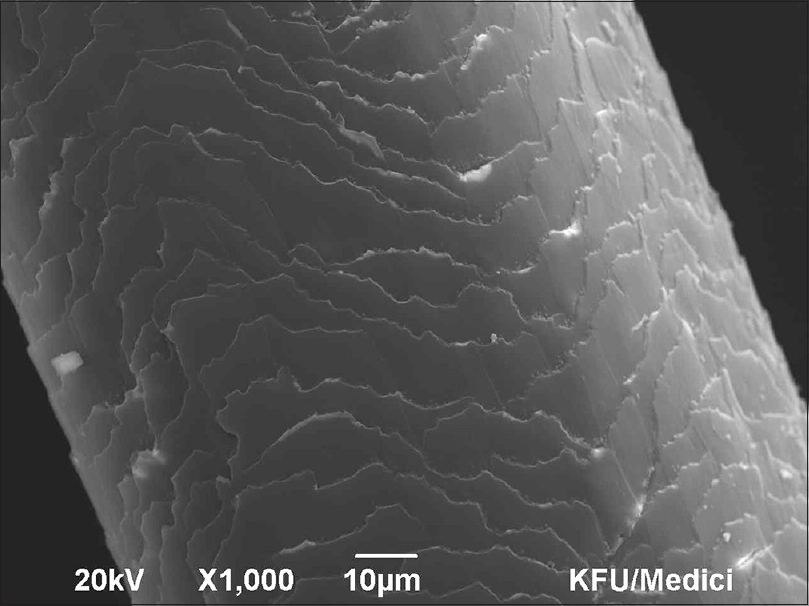
Human hairs from the scalp were attached to a double-coated carbon conductive tape, mounted on metal aluminum stubs, and sputter coated under high vacuum with the LEICA EM SCD 500 sputter coating machine using platinum as a metal target (10–20 nm thickness). Scanning of coated hair specimens was carried out using a SEM (model: JSM 6390 LA, JOEL) at 15–20 kv. Multiple sections of each shaft were scanned to ensure that changes were uniform and not isolated. Every sample was magnified with a power of at least × 1000, and was examined for different salt deposition quantities [Figure - 1] and [Figure - 2]. The SEM was combined with energy dispersive X-ray spectroscopy (EDS) for the elemental study [Figure - 3].
 |
| Figure 1: Scanning electron microscopy showing surface depositions in hard water treated hair (×1000) |
 |
| Figure 2: Scanning electron microscopy showing soft water treated hair (×1000) |
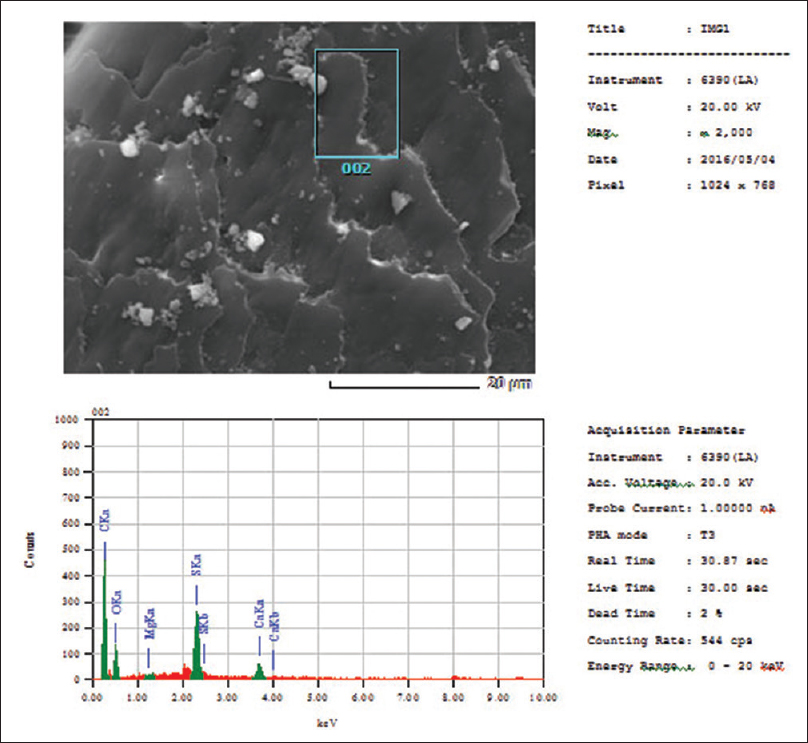 |
| Figure 3: Estimation of relative deposits on the hair shaft using scanning electron microscopy |
Grading of damage under scanning electron microscopy
The damage to hair shaft under SEM was graded into five grades (based on the grading system developed by Kim et al.[6])
Grade 0 – Virgin intact hair with regular overlay of the cuticle
Grade 1 – Irregular overlay of the cuticle without cracks or holes
Grade 2 – Severe lift-up of the cuticle with cracks or holes, but without exposure of the cortex
Grade 3 – Partial exposure of the cortex
Grade 4 – Complete exposure of the cortex.
Statistical analysis
Mann–Whitney U was used to compare the SEM scores and the relative deposition of calcium and magnesium between the study and control groups. Data analysis was done using SPSS® (SPSS for Windows, Version 16.0. Chicago, SPSS Inc).
Results
There was no statistically significant difference between the study and control groups as far as surface changes under SEM were concerned [Table - 1]. As far as the relative deposition of calcium and magnesium was concerned, there was no statistically significant difference in calcium deposition between the control and study samples (P = 0.28); however, analysis of magnesium deposition showed a significant difference between both the groups (P = 0.001), with a higher level in the samples washed with hard water [Table - 2].
Discussion
Many patients who present with breakage and brittleness of hair implicate hardness of water to be the primary underlying reason.[4] The normal tap water supply in our area is significantly hard.[1] Establishing a significant correlation between the normally used hard water and structural changes in the hair shaft would help us improve the management of patients with related complaints. Our study, unlike previous studies, attempted to simulate real-life usage – with the normally used tap water and exposure times simulating a normal shower. Our study, similar to the study by Srinivasan and Chakravarthy Rangachari, showed a statistically significantly higher deposition of magnesium salts in the samples treated with hard water; however, this was not associated with any significant difference in terms of hair shaft damage.[5] Combining our results with the results of the study by Srinivasan et al., (which found no significant differences in the elasticity and tensile strength of hair treated with hard water and soft water), we postulate that continued use of hard water might lead to an increased deposition of salts on hair surface, however, this by itself may not lead to significant brittleness or hair breakage.[4] However, another study by Luqman et al., in a sample of 76 volunteers, suggested that tensile strength might indeed be affected by the hardness of water.[7] In this study, the tensile strength of hair was significantly reduced in hair treated with hard water compared to soft water.[7] It has been postulated that long-term deposition of salts on the hair shaft may lead to an abrasive action on the hair shaft leading to surface damage, water loss, and eventually decreased thickness.[5] The significance of the relatively higher deposition of magnesium salts is not clear. A study by Cotton et al. suggested that magnesium is necessary for hair growth based on the fact that the concentration of magnesium in the scalp hair of patients with alopecia areata was lower than normal; however, there is very little data providing significant insights on how magnesium salts deposited on the surface of the hair shaft can affect structural properties of the hair.[8]
Limitations
This study has some significant limitations. The small sample size is a major limitation in our study. The absence of a pretreatment analysis of the hair shaft and mineral deposition was another limitation. Our study did not find significant surface damage on SEM. We assume that this could be because of two reasons – first we used normal tap water and second we used a short duration of exposure of only 3 weeks for assessment. Moreover, the possibility of oxidative damage to the hair shaft secondary to calcium and magnesium absorption is influenced by other factors such as pH, which was not considered in our study.[2],[3] We also did not attempt to correlate our findings with tensile strength/elasticity.
Conclusions
Hard water may be associated with increased deposits on the hair shaft surface, but this does not necessarily translate into evident structural surface changes, as evidenced by SEM. Studies with a larger sample size and a longer duration of study under real-life simulating set-ups are warranted to assess hair shaft damage caused by the hardness of water.
Acknowledgments
- Deanship of Scientific Research, King Faisal University, Hofuf, Saudi Arabia
- Hussain Alali Hospital and Research Center, Hofuf, Saudi Arabia
- Walid N. AlMelhim, Department of Biomedical Sciences, College of Medicine, King Faisal University, Hofuf, Saudi Arabia
- Hani M. AlRasasi, Department of Biomedical Sciences, College of Medicine, King Faisal University, Hofuf, Saudi Arabia.
Financial support and sponsorship
This study was funded by the Deanship of Scientific Research (student research grant), King Faisal University, Saudi Arabia.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Al-Zarah AI. Evaluation of household drinking water quality in Al-Ahsa city, Saudi Arabia. Res J Environ Sci 2014;8:62-77.
[Google Scholar]
|
| 2. |
Evans AO, Marsh JM, Wickett RR. The uptake of water hardness metals by human hair. J Cosmet Sci 2011;62:383-91.
[Google Scholar]
|
| 3. |
Evans AO, Marsh JM, Wickett RR. The structural implications of water hardness metal uptake by human hair. Int J Cosmet Sci 2011;33:477-82.
[Google Scholar]
|
| 4. |
Srinivasan G, Srinivas CR, Mathew AC, Duraiswami D. Effects of hard water on hair. Int J Trichology 2013;5:137-9.
[Google Scholar]
|
| 5. |
Srinivasan G, Chakravarthy Rangachari S. Scanning electron microscopy of hair treated in hard water. Int J Dermatol 2016;55:e344-6.
[Google Scholar]
|
| 6. |
Kim YD, Jeon SY, Ji JH, Lee WS. Development of a classification system for extrinsic hair damage: Standard grading of electron microscopic findings of damaged hairs. Am J Dermatopathol 2010;32:432-8.
[Google Scholar]
|
| 7. |
Luqman MW, Ali R, Khan Z, Ramzan MH, Hanan F, Javaid U. Effect of topical application of hard water in weakening of hair in men. J Pak Med Assoc 2016;66:1132-6.
[Google Scholar]
|
| 8. |
Cotton DW, Porters JE, Spruit D. Magnesium content of the hair in alopecia areata atopica. Dermatologica 1976;152:60-2.
[Google Scholar]
|
Fulltext Views
3,043
PDF downloads
1,063





