Translate this page into:
Segmental vitiligo: A randomized controlled trial to evaluate efficacy and safety of 0.1% tacrolimus ointment vs 0.05% fluticasone propionate cream
Correspondence Address:
Binod K Khaitan
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi - 110 029
India
| How to cite this article: Kathuria S, Khaitan BK, Ramam M, Sharma VK. Segmental vitiligo: A randomized controlled trial to evaluate efficacy and safety of 0.1% tacrolimus ointment vs 0.05% fluticasone propionate cream. Indian J Dermatol Venereol Leprol 2012;78:68-73 |
Abstract
Background: Segmental vitiligo is a small subset of vitiligo which responds very well to surgical therapy, but the role of medical treatment is not very well defined. Aim: To compare the efficacy and safety of 0.1% tacrolimus ointment versus 0.05% fluticasone propionate cream in patients of segmental vitiligo. Methods: A randomized control trial was conducted in a tertiary care hospital on 60 consecutive patients with segmental vitiligo. Patients with segmental vitiligo exclusively or along with focal vitiligo, untreated or had not taken any topical treatment in previous 1 month or systemic treatment in previous 2 months, from May 2005 to January 2007, were block randomized into two groups. Children <5 years, pregnant and lactating women, and patients with known hypersensitivity to either drug and with associated multiple lesions of vitiligo were excluded. Group A (n = 29) patients were treated with tacrolimus 0.1% ointment twice daily and group B (n = 31) patients were treated with 0.05% of fluticasone cream once daily for 6 months. Response and side effects were recorded clinically and by photographic comparison. Results: Nineteen patients treated with tacrolimus and 21 patients treated with fluticasone completed the treatment with median repigmentation of 15% and 5%, respectively, at 6 months (P = 0.38). Transient side effects limited to the application site were observed. Conclusions: Both tacrolimus and fluticasone propionate produce variable but overall unsatisfactory repigmentation in segmental vitiligo.Introduction
Vitiligo is an acquired pigmentary disorder presenting as hypopigmented or depigmented macules and affects 0.5-2% of the population worldwide. [1] Segmental vitiligo has depigmented macules arranged in a dermatomal or quasi-dermatomal distribution, which does not cross the midline and is usually unresponsive to medical treatment. [2],[3] Tacrolimus and topical corticosteroids are effective in treating vitiligo, [4],[5],[6],[7],[8],[9] but there are not many studies conducted on segmental vitiligo. We conducted this study to compare the efficacy and safety of 0.1% tacrolimus ointment versus 0.05% fluticasone propionate cream in segmental vitiligo.
Methods
Participants
This study was an open-labeled pilot study conducted on 60 consecutive patients of segmental vitiligo, presenting to us in the dermatology outpatient department at All India Institute of Medical Sciences (AIIMS), New Delhi, from May 2005 to January 2007. Patients with segmental vitiligo who were untreated, had not taken any topical treatment in previous 1 month or systemic treatment in previous 2 months were included. Segmental vitiligo was diagnosed clinically by two independent observers (SK and BKK/MR) on the basis of localized depigmented macules arranged in a particular configuration. Patients having segmental vitiligo with additional one or two small lesions of focal vitiligo were also included. Children below 5 years of age, pregnant and lactating women, patients with known hypersensitivity to either tacrolimus or fluticasone or patients with segmental vitiligo having multiple lesions of other types of vitiligo were excluded.
Pretreatment evaluation
A detailed history, cutaneous examination and baseline investigations (complete blood count, fasting and postprandial blood sugar, biochemical tests for liver and kidney function, urine and stool routine and microscopy, chest X-ray) were carried out. Baseline clinical photograph was recorded. A written informed consent was taken from the patients or parents in case of a child.
Intervention
Patients were randomized into two groups: group A and group B. Patients in group A applied either 0.1% tacrolimus ointment twice daily and in group B applied 0.05% fluticasone propionate cream once daily for 6 months. Repigmentation was assessed subjectively every month and with photograph every 2 months by two observers (SK and BKK/MR).
Hypothesis
The aim was to compare the efficacy and side effects of 0.1% tacrolimus ointment with that of 0.05% fluticasone propionate cream. It was hypothesized that there was no difference in the efficacy and side effects of 0.1% tacrolimus ointment and 0.05% fluticasone propionate cream.
Outcomes
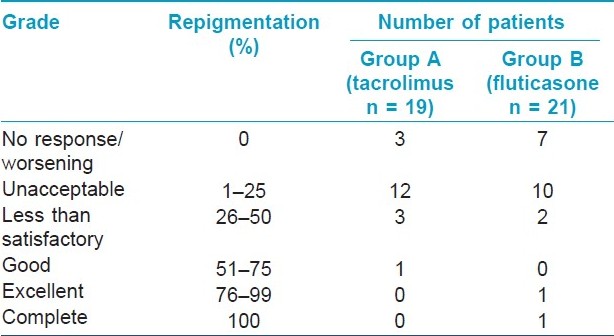
The percentage repigmentation of skin and hair, pattern of repigmentation and side effects were assessed subjectively. At the end of 6 months, pigmentation response was calculated as the percentage of total lesional area of all the macules within the segment showing repigmentation and was graded as follows:
0: no response or worsening (non-responders)
1-25%: unacceptable
26-50%: less than satisfactory
51-75%: good
76-99%: excellent
100%: complete
Treatment failure was classified as <50% repigmentation after 6 months. The color match at 6 months was graded as excellent, good and poor.
Sample size and power
To have power of the study as 80% and type I error as 5%, the sample size was calculated to be 212 patients (106 patients in each group). However, due to constraints of the study period of only 2 years in this project and segmental vitiligo being a small subset of vitiligo, the sample size was arbitrarily taken as 60 patients. The power of this study was retrospectively calculated to be 2.3%.
Randomization
Sequence generation: The random allocation sequence was computer generated and consisted of series of group number (either 1 = A or 2 = B) for each consecutive patient. Block randomization method was used and each block was of 10 patients.
Allocation concealment: The group number was written on a paper and sealed into separate envelopes which bore the number of the corresponding patient, by a departmental colleague not associated with the study. None of the investigators were involved in the generation of random allocation sequence or preparing the envelopes.
Implementation: The patients were enrolled by the primary investigator (SK). The envelope was opened and the patient was randomized into either group.
Statistical methods
STATA 9.0 (STATACorp LP) was used for statistical analysis. Since the data were skewed, the median and range were considered for each variable. The differences in the variables were compared using Wilcoxon rank sum test. P value less than 0.05 was considered statistically significant.
Ethics committee clearance
The study protocol was approved by the Ethics Committee, AIIMS, in May 2005. The study protocol was not registered as it was initiated in May 2005, when it was not mandatory to register trials.
Results
A total of 60 patients were recruited. The progress of the study through various phases is depicted in [Figure - 1].
 |
| Figure 1: Flow diagram of the progress of the study through various phases |
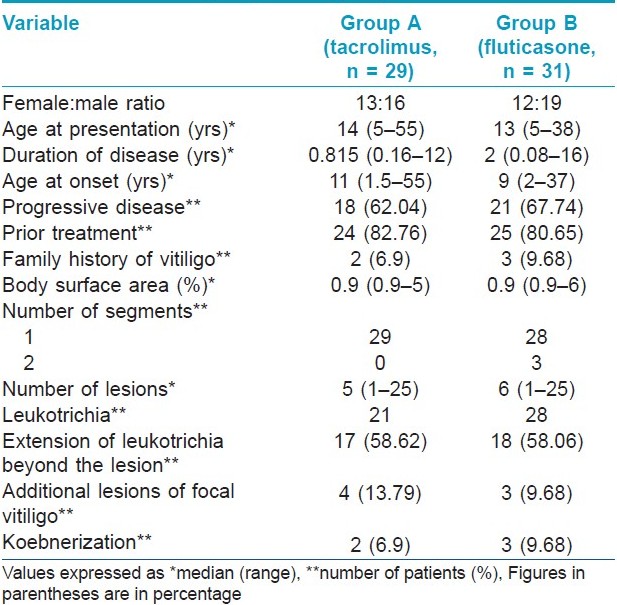
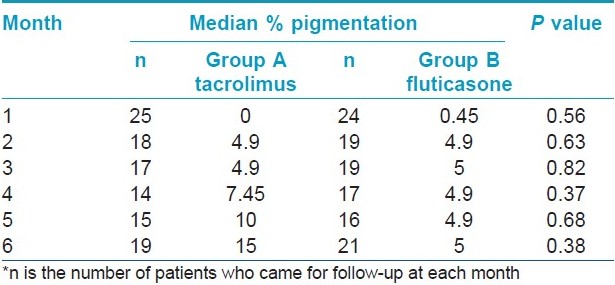
The clinical characteristics of patients in both the groups are summarized in [Table - 1]. Repigmentation at some point of time during the study was seen in 18 and 17 patients in groups A and B, respectively. Study duration was completed by 19 patients in group A and 21 patients in group B. The median area showing repigmentation with tacrolimus at the end of 6 months was 15% (range 0-60%), whereas the median area showing repigmentation with fluticasone propionate was 5% (range 0-100%) (P = 0.38) [Figure - 2],[Figure - 3],[Figure - 4],[Figure - 5]. The repigmentation at various months and grade of repigmentation at 6 months are given in [Table - 2] and [Table - 3], respectively. Repigmentation of leukotrichia was not seen in any patient in either of the groups. There did not seem to be any difference in the repigmentation response with respect to family history, site and disease duration; however, P value could not be calculated due to small number of patients in each group. The color match was good or excellent in both the groups.
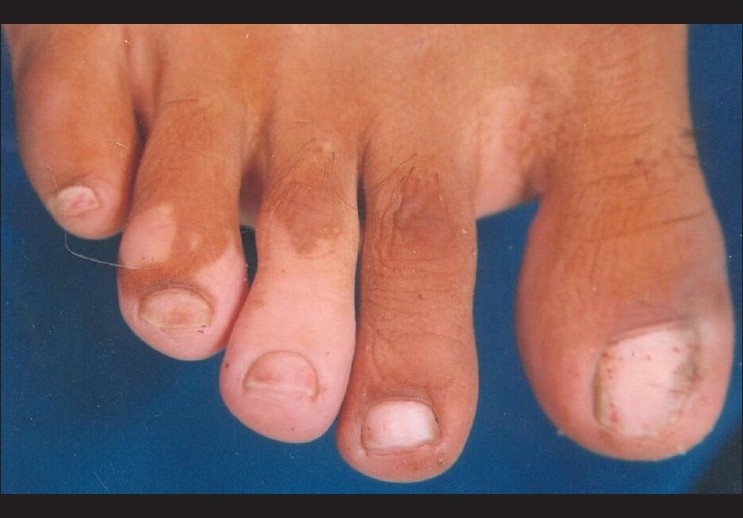 |
| Figure 2: Segmental vitiligo of the feet at baseline |
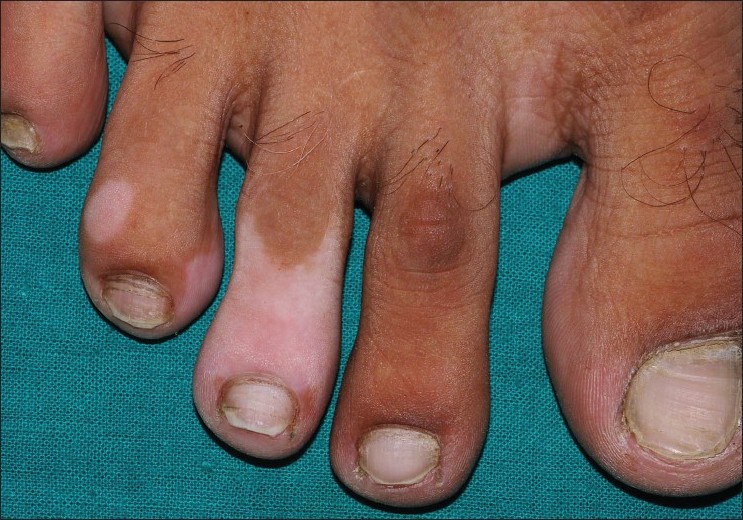 |
| Figure 3: Minimal repigmentation with tacrolimus at 6 months |
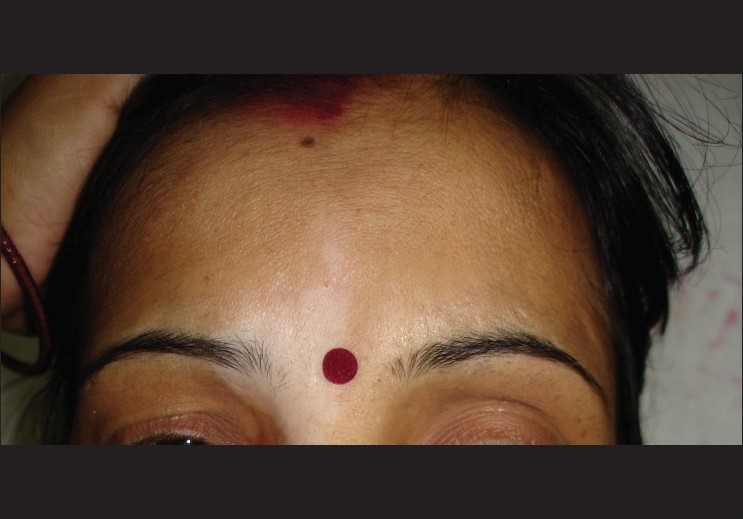 |
| Figure 4: Segmental vitiligo of the face at baseline |
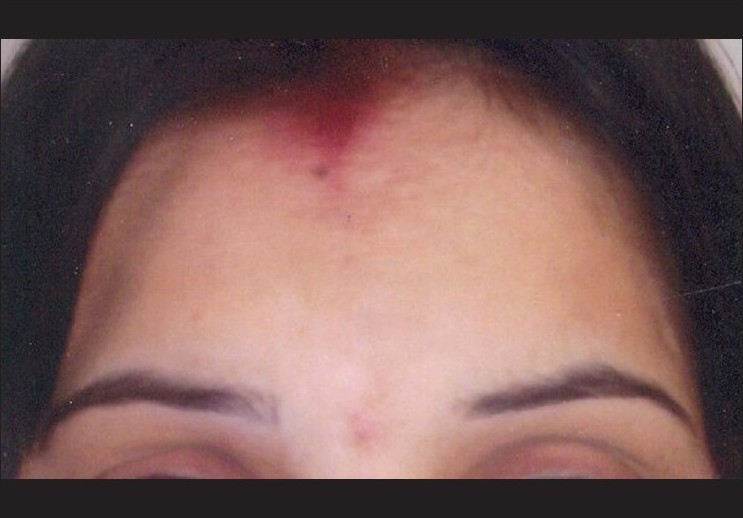 |
| Figure 5: Near complete repigmentation with fluticasone at 6 months |
Pattern of repigmentation was assessed in 18 and 17 patients showing repigmentation at some point of time during the study. Peripheral repigmentation alone or in combination with central or diffuse repigmentation was the commonest type, seen in 16/18 (88.88%) patients in group A and 17/17 (100%) patients in group B. Central repigmentation alone was seen in one patient in group A and none in group B. Perifollicular pattern of repigmentation was not seen in any patient.
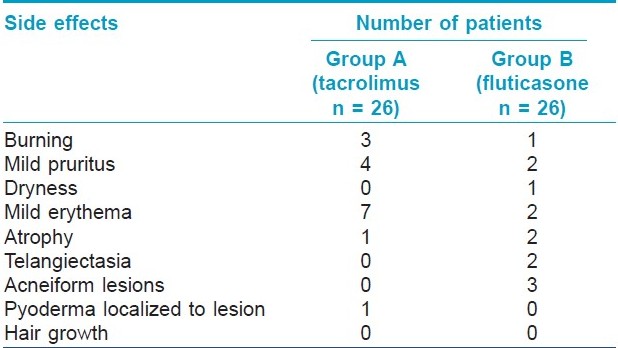
Side effects were minimal and did not warrant withdrawal from the study. [Table - 4] shows the number of patients in each group who developed side effect at any time during their treatment. All these side effects were confined to the lesion/area of application of the medicament. Twelve patients and 17 patients in groups A and B, respectively, did not have any side effects.
Discussion
Segmental vitiligo is a specific type of vitiligo considered resistant to medical treatment. [2],[3] There are several studies on topical therapy of vitiligo, but perhaps due to the unresponsiveness of segmental vitiligo to topical preparations, there are not many studies addressing the subject. [10]
The patients in both the groups had no difference with regards to the sex ratio, age at presentation, age at onset, disease duration, percentage of patients with active disease, body surface area involvement, sites involved and leukotrichia. Due to the small body surface area involvement and minimal repigmentation, only semi-quantitative assessment of repigmentation was feasible. Seven patients had one or two lesions of focal vitiligo in addition to segmental vitiligo which were treated with tacrolimus or fluticasone propionate as per their group.
The median area showing repigmentation after 6 months of treatment was 15% with tacrolimus and 5% with fluticasone propionate. In terms of number of patients showing repigmentation, patients with no repigmentation at all were more with fluticasone propionate (33.34%) as compared to tacrolimus (15.7%) at 6 months. Considering achievement of ≤25% repigmentation as unacceptable and 26-50% as unsatisfactory, it was observed that 94.7% patients in tacrolimus group and 90.4% patients in fluticasone group had such results amounting to treatment failure. Only 5.3% and 9.6% patients with tacrolimus and fluticasone, respectively, had >50% repigmentation, showing the limitation of these drugs. Repigmentation of leukotrichia did not occur in any patient. Thus, our study suggests that segmental vitiligo does not respond favorably to medical treatment with either topical tacrolimus or fluticasone except in a small percentage (<10%) of patients. Previously, Khalid and Mujtaba reported >50% repigmentation in 34.2% patients with clobetasol propionate. [10] The slightly better response in their study can be attributed to the use of a higher potency corticosteroid.
However, segmental vitiligo cannot be considered completely refractory to topical medications as mentioned previously, as only 3/19 in tacrolimus group and 7/21 in fluticasone group did not show any response even after 6 months of treatment. Of the patients who showed some repigmentation, 85% and 88.2% of group A and group B, respectively, had onset of repigmentation by the 3 rd month. So, a trial of minimum 6 months of treatment should be given before declaring either drug as ineffective. The color match was very good in all the patients.
According to Ostovari et al, the UV exposure enhances the efficacy of tacrolimus. [11] In this study, no statistically significant correlation could be made between the response to treatment and the site of involvement, disease progression or duration of disease in either group.
Parsad et al, reported diffuse repigmentation as the commonest pattern of repigmentation in segmental vitiligo. [12] In contrast, in our study, peripheral or marginal repigmentation, either alone or in combination with central repigmentation, was the commonest pattern observed with both tacrolimus and fluticasone.
More number of patients developed side effects with tacrolimus [14/26 (54%)] as compared to fluticasone [9/26 (35%)]. However, the difference was not statistically significant and the side effects were not severe enough to warrant withdrawal. Kanwar et al, [6] reported side effects like pruritus and burning sensation in 13.6% patients, which can be explained by the use of lower concentration of tacrolimus (0.03%) in contrast to 0.1% in our study. Molluscum contagiosum is an infective condition reported with tacrolimus. [13] In our study, localized pyococcal infection was the only infective side effect observed. The cause for development of these infections could be the localized immunosuppression by tacrolimus.
Khalid and Mujtaba [10] reported mild atrophy in 6 and telangiectasia in 4 out of 30 patients. In our study, we observed epidermal atrophy in two patients. The increased number of side effects in their study as compared to ours can be explained as due to the use of higher potency corticosteroid. Westerhof reported that none of their patients developed atrophy or any other side effect with the use of fluticasone propionate for 9 months. [14]
The small number of patients caused the power of the study to be low, and a follow-up period limited to 6 months in both the groups was a limitation of the study. Larger studies are recommended to find out if there are any factors which can help us decide which patient of segmental vitiligo will respond and to what extent with topical tacrolimus or fluticasone propionate. Surgical treatment produces better results in segmental vitiligo as compared to medical treatment. So, both these topical agents can also be used to get some repigmentation and reduce the total lesional area prior to surgery. These drugs may also have an advantage on the areas like eyelids, where surgical treatment is relatively difficult.
The cost of the drug becomes important in a developing economy, particularly when there is no difference in the treatment response. In our setting, tacrolimus ointment 0.1% costs about three times more than fluticasone propionate 0.05% cream. Also, as per the general guidelines, tacrolimus is applied twice a day as compared to once a day application of fluticasone, so the cost further increases.
Our study suggests that both topical tacrolimus and fluticasone have a limited efficacy in segmental vitiligo in terms of number of patients showing repigmentation and the percentage area showing repigmentation. The side effects are comparable, transient and acceptable. However, the use of either topical tacrolimus or fluticasone in segmental vitiligo cannot be completely discarded as it may have a role to play in halting further progression of the disease.
| 1. |
Lerner AB. Vitiligo. J Invest Dermatol 1959;32:285-310.
[Google Scholar]
|
| 2. |
Hann SK. Clinical features of segmental Vitiligo. In: Hann SK, Nordlund JJ, editors. Vitiligo, 1 st ed. Oxford : Blackwell Science; 2000. p. 49-60.
[Google Scholar]
|
| 3. |
Hann SK, Lee HJ. Segmental vitiligo: Clinical findings in 208 patients. J Am Acad Dermatol 1996;35:671-4.
[Google Scholar]
|
| 4. |
Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol 2002;47:489-91.
[Google Scholar]
|
| 5. |
Travis LB, Weinberg JM, Silverberg NB. Successful treatment of vitiligo with 0.1% tacrolimus ointment. Arch Dermatol 2003;139:571-4.
[Google Scholar]
|
| 6. |
Kanwar AJ, Dogra S, Parsad D. Topical tacrolimus for treatment of childhood vitiligo in Asians. Clin Exp Dermatol 2004;29:589-92.
[Google Scholar]
|
| 7. |
Silverberg NB, Lin P, Travis L, Farley-Li J, Mancini AJ, Wagner AM, et al. Tacrolimus ointment promotes repigmentation of vitiligo in children: a review of 57 cases. J Am Acad Dermatol 2004;51:760-6.
[Google Scholar]
|
| 8. |
Njoo MD, Spuls PI, Bos JD, Westerhof W, Bossuyt PM. Nonsurgical repigmentation therapies in vitiligo. Meta-analysis of the literature. Arch Dermatol 1998;134:1532-40.
[Google Scholar]
|
| 9. |
Lepe V, Moncada B, Castaneda-Cazares JP, Torres-Alvaerez MB, Ortiz CA, Torres-Rubalcava AB. A double-blind randomized trial of 0.1% tacrolimus vs 0.05% clobetasol for the treatment of childhood vitiligo. Arch Dermatol 2003;139:581-5.
[Google Scholar]
|
| 10. |
Khalid M, Mujtaba G. Response of segmental vitiligo to 0.05% clobetasol propionate cream. Int J Dermatol 1998;37:701-8.
[Google Scholar]
|
| 11. |
Ostovari N, Passeron T, Lacour JP, Ortonne JP. Lack of efficacy of tacrolimus in the treatment of vitiligo in the absence of UV-B exposure. Arch Dermatol 2006;142:252-3.
[Google Scholar]
|
| 12. |
Parsad D, Pandhi R, Dogra S, Kumar B. Clinical study of repigmentation patterns with different treatment modalities and their correlation with speed and stability of repigmentation in 352 vitiliginous patches. J Am Acad Dermatol 2004;50:63-7.
[Google Scholar]
|
| 13. |
Abu BK, Kim BD, Lee SJ, Lee SH. Molluscum contagiosum infection during the treatment of vitiligo with tacrolimus ointment. J Am Acad Dermatol 2005;52:532-3.
[Google Scholar]
|
| 14. |
Westerhof W, Neuweboer-Krobotova L, Mulder PG, Glazenberg EJ. Left-right comparison study of the combination of fluticasone-propionate and UV-A Vs either fluticasone propionate or UV-A alone for the long-term treatment of vitiligo. Arch Dermatol 1999;135:1061-6.
[Google Scholar]
|
Fulltext Views
7,817
PDF downloads
2,241





