Translate this page into:
Severe eosinophilia during anti-tumor necrosis factor-alpha therapy for psoriatic arthritis
Correspondence Address:
Giacomo Maria Guidelli
Department of Medicine, Surgery and Neurosciences, Rheumatology Unit, Siena University, Viale Bracci 1, 53100 Siena
Italy
| How to cite this article: Guidelli GM, Tenti S, Fioravanti A. Severe eosinophilia during anti-tumor necrosis factor-alpha therapy for psoriatic arthritis. Indian J Dermatol Venereol Leprol 2014;80:187-189 |
Sir,
Since 1999, tumor necrosis factor-alpha (TNF-alpha) antagonists are being commonly used in the treatment of rheumatological, dermatological and gastroenterological autoimmune diseases.
Despite their striking effectiveness and an acceptable toxicity profile, several side effects have been reported, including increased risk of serious infections due to both commensal and opportunistic microorganisms, lymphoma and solid tumors and adverse dermatological reactions. [1]
Here, we briefly report the case of a man with psoriatic arthritis who developed blood eosinophilia first during treatment with etanercept and then with adalimumab.
A 59-year-old Caucasian man had been diagnosed with psoriatic arthritis 15 years ago. Initially, during the early years of the disease, he reported being treated with oral corticosteroids and sulfasalazine and then with methotrexate.
In 2010, the patient still had active arthritis, with a psoriasis area severity index (PASI) score of 6.7; hence, he had been receiving combination therapy with etanercept, 25 mg twice a week and oral methylprednisolone, 4 mg/day. Joint involvement rapidly improved while the PASI score did not change during treatment [Figure - 1]. However, routine blood analysis after the first month of therapy, which included a total of eight injections, revealed eosinophilia of 24% (normal value: 0-6%) with a total white blood cell count of 8.30 × 10 3 /mm 3 ; [Figure - 1]. Other causes of eosinophilia, such as parasitic infections, allergies and non-Hodgkin′s lymphoma were excluded by complete hematological, allergological and microbiological work-up. On this basis, etanercept was withdrawn and laboratory findings returned to normal within 30 days.
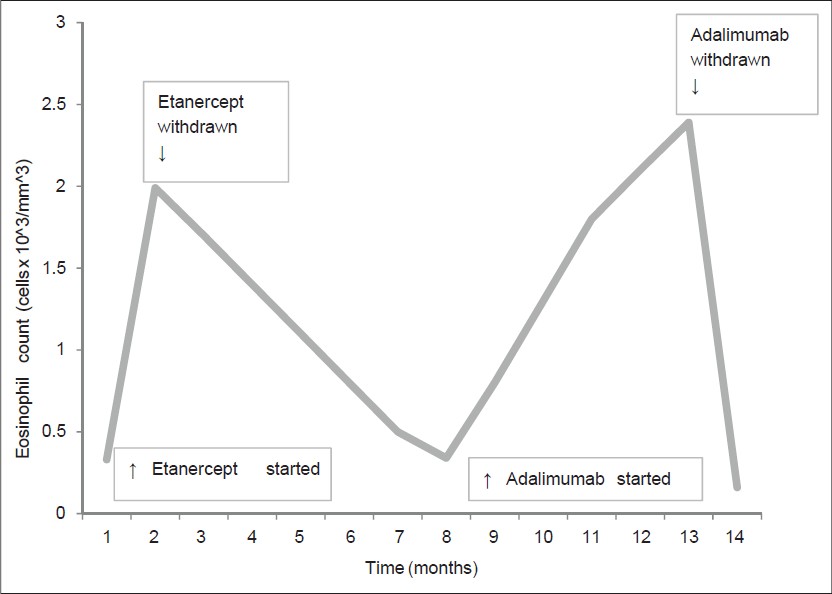 |
| Figure 1: Variation in eosinophil counts over time |
After 6 months of treatment with oral corticosteroids alone, the patient was seen in our rheumatologic outpatient clinic for a relapse of the arthritis. Clinically, the patient showed joint effusion of his right knee, wrists and small joints of the left hand; psoriasis plaques were still present on his elbows (PASI score: 5.9).
Laboratory tests showed anemia with features of chronic disease with a hemoglobin level of 11.2 g/dl, with low serum iron and high ferritin levels, a raised erythrocyte sedimentation rate of 58 mm/h (normal value: 0-35) and C-reactive protein of 2.7 mg/dl (normal value: <0.5). X-rays showed erosive lesions in the wrist and metacarpophalangeal joints, bilaterally. The patient was treated with subcutaneous injections of adalimumab, 40 mg every 14 days, which resulted in a rapid clinical improvement of the tender and swollen joints.
However, after 5 months, the eosinophil count rose up to 38.6% of a total white blood cell count of 6.2 × 10 3 /mm 3 [Figure - 1].
As with the previous episode of eosinophilia following etanercept therapy, a parasite screen was negative, total IgE remained normal and no signs of a hematological disorder were found. After discontinuation of adalimumab, eosinophilia disappeared within 40 days [Figure - 1].
Currently, the patient is being treated with leflunomide, 20 mg/day, a conventional disease-modifying anti-rheumatic drug, without relapse of the arthritis.
The introduction of biologic drugs such as TNF-alpha inhibitors has improved treatment efficacy in several chronic inflammatory disorders including psoriatic arthritis. However, the challenge for clinicians is to evaluate the potential drug-related side-effects.
In our case, the link between the administration of etanercept and then adalimumab with the increase in eosinophil count is clear.
Some authors have previously described eosinophilic pathology associated with anti TNF-alpha drugs. Boura et al.[2] and Winfield et al. [3] described the development of eosinophilic cellulitis (Wells syndrome) after administration of adalimumab and etanercept. Cancelliere et al.[4] reported the case of a patient with rheumatoid arthritis who developed subacute prurigo with eosinophilia after infliximab and etanercept therapy. Furthermore, a transient blood eosinophilia during adalimumab administration for acrodermatitis continua of Hallopeau has been described by Vester et al.[1] More recently, Malisiewicz et al.[5] showed the development of isolated eosinophilia in three patients with psoriasis (two of who also had joint involvement) during TNF blockade.
In the eHealthMe database, on October 6, 2012, among 126860 people reporting to have side effects when taking adalimumab, 74 (0.06%) had eosinophilia. At the same date, with regard to etanercept, 60 (0.04%) patients among 166562 people reporting side-effects reported the development of eosinophilia. The majority of these patients were women in the fifth decade of life.
The mechanism by which TNF-alpha inhibition leads to blood eosinophilia remains unclear. It has been hypothesized that the generation of IgE-class-switched antibodies might determine IgE-mediated drug hypersensitivity and subsequent eosinophilia; furthermore, anti-TNF-alpha agents, especially adalimumab, might also be able to influence eosinophil apoptosis. [6]
This case highlights the importance of early identification and reporting of drug side-effects, such as eosinophilia, which, however, remains a rare adverse event of TNF-alpha blockers. This side effect is not often clinically detectable, but it could be responsible for severe organ damage due to the release of toxic granule proteins such as eosinophil-derived neurotoxin, eosinophilic cationic protein, eosinophil peroxidase and eosinophil major basic protein.
Our case and some cases reported in the literature suggest that after the development of eosinophilia, clinicians should switch therapy, not from one TNF-alpha inhibitor to another one, but to other drugs with a different mechanism of action.
| 1. |
Vester K, Rüger RD, Harth W, Simon JC. Transient blood eosinophilia during treatment with adalimumab. J Eur Acad Dermatol Venereol 2012;26:924-5.
[Google Scholar]
|
| 2. |
Boura P, Sarantopoulos A, Lefaki I, Skendros P, Papadopoulos P. Eosinophilic cellulitis (Wells' syndrome) as a cutaneous reaction to the administration of adalimumab. Ann Rheum Dis 2006;65:839-40.
[Google Scholar]
|
| 3. |
Winfield H, Lain E, Horn T, Hoskyn J. Eosinophilic cellulitis-like reaction to subcutaneous etanercept injection. Arch Dermatol 2006;142:218-20.
[Google Scholar]
|
| 4. |
Cancelliere N, Barranco P, Vidaurrázaga C, Benito DM, Quirce S. Subacute prurigo and eosinophilia in a patient with rheumatoid arthritis receiving infliximab and etanercept. J Investig Allergol Clin Immunol 2011;21:248-9.
[Google Scholar]
|
| 5. |
Malisiewicz B, Murer C, Pachlopnik Schmid J, French LE, Schmid-Grendelmeier P, Navarini AA. Eosinophilia during psoriasis treatment with TNF antagonists. Dermatology 2011;223:311-5.
[Google Scholar]
|
| 6. |
Simon HU, Yousefi S, Dibbert B, Levi-Schaffer F, Blaser K. Anti-apoptotic signals of granulocyte-macrophage colony-stimulating factor are transduced via Jak2 tyrosine kinase in eosinophils. Eur J Immunol 1997;27:3536-9.
[Google Scholar]
|
Fulltext Views
3,872
PDF downloads
1,445





