Translate this page into:
Single-nucleotide polymorphism and haplotype analysis of macrophage migration inhibitory factor gene and its correlation with serum macrophage migration inhibitory factor levels in North Indian psoriatic patients with moderate disease severity: A cross-sectional study
-
Received: ,
Accepted: ,
How to cite this article: Chhabra S, Banerjee N, Narang T, Sood S, Bishnoi A, Goel S, et al. Single-nucleotide polymorphism and haplotype analysis of macrophage migration inhibitory factor gene and its correlation with serum macrophage migration inhibitory factor levels in North Indian psoriatic patients with moderate disease severity: A cross-sectional study. Indian J Dermatol Venereol Leprol 2023;89:247-53.
Abstract
Background:
Psoriasis is associated with significant morbidity and impaired quality of life. Identification of the host genes that influence disease susceptibility and can potentially guide future, targeted therapy is the need of the hour.
Aims:
The aim of the study was to investigate the associations of macrophage migration inhibitory factor (MIF) gene polymorphisms, that is, a 5–8-CATT tetra nucleotide repeats at -794 (-794*CATT5–8) and a single-nucleotide polymorphism at -173 (-173*G/C) with the risk of chronic plaque psoriasis and to observe the correlation, if any, of disease determinants with genetic functional variants and circulating MIF levels.
Methods:
Five hundred and seventeen individuals (265 psoriasis patients and 252 controls) were genotyped for MIF gene polymorphisms. Data were analyzed with respect to disease susceptibility, serum MIF levels, disease severity, age at onset, disease duration and presence of comorbidities.
Results:
The presence of co-morbidities was more frequently noted in patients with late onset disease (P = 0.01). No statistically significant differences were observed either in genotype (P = 0.680) or allele frequency (P = 0.69) with respect to distribution of MIF-173*G/C polymorphism between patients and controls. The frequencies of genotypes -794*CATT 5/7 and 7/7 were significantly lower in patients (P = 0.027* and 0.038*, respectively). CATT*5/MIF-173*C haplotype occurred at a higher frequency in patients (odds ratio 3.03, 95% confidence intervals 1.09–8.47, P = 0.02). The mean serum MIF levels were significantly higher in patients as compared to controls (P < 0.001). The presence of either extended MIF -794*CATT repeats or C allele did not reveal any significant association with serum MIF levels or age at onset. Analysis of effect of various disease determinants revealed no significant association with genetic variants and serum MIF levels.
Limitations:
The lesional expression of MIF could not be studied.
Conclusion:
Our results showed that CATT*5/MIF-173*C haplotype is associated with increased susceptibility to psoriasis vulgaris.
Keywords
Macrophage migration inhibitory factor
psoriasis
single-nucleotide polymorphism
Introduction
Psoriasis is a common chronic inflammatory proliferative skin disease with prevalence varying from region to region due to various environmental and genetic factors.1,2 Macrophage migration inhibitory factor (MIF) is a potent pro-inflammatory cytokine involved in the pathogenesis of many inflammatory diseases, including psoriasis; a role suggested by its activity profile which includes induction of tumor necrosis factor-α secretion and promotion of T-cell activation.3-5 Polymorphisms in the promoter region of MIF gene, that is, CATT tetranucleotide repeat at -794 (rs5844572) or guanine (G) to cytosine (C) transition at -173 (-173*G/C, rs755622) have been reported to be associated with increased susceptibility to, or severity of, numerous chronic inflammatory, and autoimmune disorders in different populations.4-16 Given these data, the pharmacological or immunological modulation of MIF activity might offer new therapeutic options for patients suffering from these diseases.6
We planned this study to see whether MIF gene polymorphisms affect the susceptibility to and severity of chronic plaque psoriasis. Few studies have demonstrated MIF gene polymorphisms with psoriasis in different ethnic populations across the globe; however, no studies have documented the impact of MIF promoter variations on the risk of chronic plaque psoriasis in North Indian population.17,18 The main objective was to investigate the association between these two functional MIF polymorphisms, that is, -794*CATT5–8 and -173*G/C and chronic plaque psoriasis and to observe a correlation, if any, between susceptibility and genetic functional MIF variants.
Methods
A total of 265 consenting patients of chronic plaque psoriasis were included in the study. None of the recruited patients had received any systemic or topical treatment for the last 4 weeks and 2 weeks respectively, before enrolment. None of the patients had any significant renal, hepatic, or other systemic disease (confounding conditions such as atopic dermatitis, lupus, systemic sclerosis and rheumatoid arthritis that can elevate MIF levels were excluded from the study). The disease severity was assessed by calculating the psoriasis area severity index (PASI) scores. Peripheral blood samples were obtained from patients and 252 age, sex, ethnically matched, healthy and unrelated controls. Genomic DNA was isolated from EDTA blood and stored. Serum MIF levels were measured by enzyme linked immunosorbent assay, ELISA (R&D Systems, Quantikine). The study was approved by the institutional ethics committee PGIMER, Chandigarh (PGI/IEC/2011/606-07 dated 05/12/2011).
Migration inhibitory factor-173*G/C genotyping (rs755622)
This single-nucleotide polymorphism was genotyped using polymerase chain reaction, followed by restriction fragment length analysis.
Migration inhibitory factor-794*CATT5-8 genotyping (rs5844572)
This polymorphism was genotyped using polymerase chain reaction, followed by capillary gel electrophoresis.
Statistical analysis
The sample-size was determined based on the frequency of single-nucleotide polymorphisms as reported in literature and using software Quanto version 1.2, (http://hydra.usc.edu/gxe). Total sample-size calculated was 500 (250 patients and 250 controls) with 5% alpha error and 95% power. Considering a 6% drop out, this translated to 265 patients. The Hardy Weinberg equilibrium was analyzed for each polymorphism online, using http://www.oege.org/software/hwe-mr-calc.shtml. Statistical analysis was performed using IBMSPSS (Statistical Package for the Social Sciences) version 20. All statistical tests were two-sided and were performed at a significance level of α=0.05. Tests of association were performed on full genotype and following simplification to 55, 5X or XX, where X = CATT6-8, as previously described.15,19-21 The haplotype analysis was performed using PHASE, version v2.1 (http://www.stat.washington.edu/stephens/software.html), Haplo.score and Haplo.glm: http://www.mayo.edu/research/labs/statistical-genetics-genetic-epidemiology/software).
Logistic regression analysis was performed to test for any association between the genetic variants and various disease determinants such as sex (male vs. female), age at onset {early (≤ 40 years) vs. late (>40 years)}, disease duration {short (≤ 5 years) vs. long (>5 years)}, disease severity {mild-to-moderate (PASI≤10) vs. severe (PASI>10)}, presence of comorbidities (present vs. absent) and serum MIF levels. Receiver-operating characteristics (ROC) curve analysis was performed to calculate the cutoff values of the serum MIF levels and to predict their specificity and sensitivity.
Results
Patient characteristics
Males outnumbered females by a ratio of 2.6:1. The mean age of onset of psoriasis was 32.6±15.5 years. The majority of patients (n=185, 69.8%) had early onset (≤40 years) disease. Disease associated comorbidities were seen in 70 (26.4%) patients, while 152 (57.4%) had disease exacerbation at the time of presentation. The demographic characteristics of both groups (patients and controls) are summarized in Table 1.
| Variables | Patients (n=265) | Controls (n=252) | P |
|---|---|---|---|
| Age at onset (years) (mean, range) | 32.6±15.5, 1–73 | NA | |
| Male: female ratio | 2.6 | 2.1 | 0.216 |
| Disease onset (years) | |||
| <40 | 24.2±9, 1–40 | NA | |
| >40 | 51.9±8.8, 41–73 | ||
| Age of onset in males (years) | 32.96±15 | NA | 0.545 |
| Age of onset in females (years) | 31.65±16.8 | NA | |
| Psoriasis area severity index (mean, range) | 6.04±6.2, 0–59 | NA | |
| Serum MIF levels (mean)* (ng/ml) | 15.68±5.86 | 7.75±2.72 | <0.001 |
| Duration of psoriasis in years (mean, range) | 8.7±7.1, 1 month–40 years | NA | |
| Number of patients with comorbidity | 70 (26.4%) | NA | |
| Body surface area (mean, range) | 10.01±11.54, 0%–90% | NA |
A significant association was observed between age at onset and presence of comorbidities (P = 0.01**) adjusted for age and sex. The presence of comorbidities was more frequently noted in patients with late onset disease (odds ratio=2.579).
Analysis of allele and genotype frequencies of migration inhibitory factor-173*G/C and migration inhibitory factor-794*CATT5–8 polymorphisms
There were no deviations from Hardy Weinberg equilibrium in patients or controls for either MIF-173 or the CATT repeat polymorphism (p>0.05). No linkage disequilibrium was observed between the studied alleles. GG genotype was most commonly encountered among both patients (144, 54.3 %) and controls (139, 55.1%). MIF-173 genotype and allele frequencies were comparable between the two groups (P = 0.680) {G/C vs. G/G, Odds ratio=1.11, 95% confidence intervals (0.77–1.59), P = 0.55; C/C vs. G/G, Odds ratio=0.51, 95% confidence intervals (0.21–1.25), P = 0.13}. Carriage of the -173*C polymorphism (GC+CC vs.GG) in patients did not reveal an association with disease (Odds ratio= 1.03, 95% confidence intervals 0.73–1.46, P = 0.85). The frequency of G allele was over-represented in both patients (76%) and controls (75 %). No significant association was detected between MIF-173 SNP and risk of psoriasis vulgaris using all models of inheritance [Table 2].
| Polymorphism | Genotypes and alleles | Patients (n=265), n (%) | Controls (n=252), n (%) | OR (CI) | P |
|---|---|---|---|---|---|
| −173 G/C | G | 401 (75.6) | 376 (74.6) | Reference | Reference |
| C | 129 (24.3) | 128 (25.3) | 0.94 (0.71–1.25) | 0.69 | |
| G/G | 144 (54.3) | 139 (55.1) | Reference | Reference | |
| G/C | 113 (46.6) | 98 (38.9) | 1.11 (0.77–1.59) | 0.55 | |
| C/C | 8 (3.0) | 15 (5.9) | 0.51 (0.21–1.25) | 0.13 | |
| G/C+C/C | 121 (45.6) | 113 (44.8) | 1.03 (0.73–1.46) | 0.85 | |
| −794 CATT | 5 | 140 (26.4) | 109 (21.6) | Reference | Reference |
| 6 | 330 (62.2) | 308 (61.1) | 0.83 (0.62–1.12) | 0.23 | |
| 7 | 59 (11.1) | 87 (17.3) | 0.52 (0.34–0.79) | 0.002 | |
| 8 | 1 (0.2) | - | - | - | |
| Grouped | |||||
| 5 | 140 (26.4) | 109 (21.6) | Reference | Reference | |
| >5 (X allele) | 390 (73.5) | 395 (78.3) | 0.76 (0.57–1.02) | 0.080 | |
| 5/5 | 20 (7.5) | 11 (4.4) | Reference | Reference | |
| 6/6 | 101 (38.1) | 100 (39.7) | 0.55 (0.25–1.21) | 0.17 | |
| 7/7 | 3 (1.1) | 9 (3.6) | 0.18 (0.04–0.82) | 0.038 | |
| 5/6 | 87 (32.8) | 63 (25) | 0.75 (0.33–1.69) | 0.55 | |
| 5/7 | 13 (4.9) | 24 (9.5) | 0.29 (0.10–0.80) | 0.027 | |
| 6/7 | 40 (15.1) | 45 (18) | 0.48 (0.20–1.14) | 0.14 | |
| 6/8 | 1 (0.4) | - | - | - | |
| 5/5+5/6+6/6 | 208 (78.5) | 174 (69.0) | Reference | Reference | |
| 5/7+6/7+7/7+6/8 | 57 (21.5) | 78 (31.0) | 0.61 (0.41–0.90) | 0.01 |
OR: Odds ratio, CI: Confidence interval
Genotype frequencies of CATT repeats element were compared between both groups (P = 0.033*). The most commonly observed genotype among patients and controls was 6/6 [Figure 1]. The frequency of -794*CATT5/7 and -794*CATT7/7 genotypes were significantly lower in patients (P = 0.027* and 0.038*, respectively), compared to the controls. When carriage of MIF CATT*7 (5/7+ 6/7 + 7/7 vs. 5/5 + 5/6 + 6/6) was compared between patients and controls, statistically significant differences were noted, with carriage of MIF CATT7 being lower in patients (P = 0.01*). The allelic distribution shows that the allele -794CATT*7 was significantly underrepresented in patients {Odds ratio=0.52, 95% confidence intervals (0.34–0.79), P = 0.002*}. None of the CATT alleles (5, 6, 7) individually were found to be associated with increased risk of developing disease (p>0.05). We grouped MIF-794 CATT6-8 repeats and termed them X allele. X allele frequency was compared between two groups and it showed an almost equal distribution (P = 0.080).

- Distribution of MIF-794*CATT tetranucleotide repeats (genotypes) in patients and healthy controls
Migration inhibitory factor haplotype analyses
The haplotype 6G was the most frequent amongst both patients and controls [Table 3]. The CATT5-MIF-173*C haplotype was observed to be significantly higher in patients with an Odds ratio of 3.03 (95% confidence intervals =1.09– 8.47). A significant evidence for association with disease was observed (P = 0.02), thereby suggesting the importance of this haplotype in susceptibility to psoriasis in our cohort.
| Haplotypes | Psoriasis patients (2 n=530), n(%) | Healthy controls (2 n=504), n(%) | OR (95% CI) |
P |
|---|---|---|---|---|
| MIF CATT5-173G | 122 (23) | 103 (20.4) | Reference | Reference |
| MIF CATT5-173C | 18 (3.3) | 5 (1) | 3.03 (1.09–8.47) | 0.02* |
| MIF CATT6-173G | 273 (51.5) | 255 (50.6) | 0.90 (0.66–1.23) | 0.57 |
| MIF CATT6-173C | 57 (10.8) | 54 (10.7) | 0.89 (0.56–1.40) | 0.64 |
| MIF CATT7-173G | 6 (1.1) | 17 (3.4) | 0.29 (0.11–0.78) | 0.01 |
| MIF CATT7-173C | 53 (10.0) | 71 (14.0) | 0.63 (0.40–0.98) | 0.04 |
| MIF CATT8-173C | 1 (0.2) | 0 | NA | NA |
The MIF CATT5-173G haplotype was selected as the baseline for comparisons (no associated OR, 95% CI or P value). NA: Not applicable (no test was done for uncommon haplotypes because the low frequencies are not reliable for comparison), OR: Odds ratio, CI: Confidence interval, MIF: Migration inhibitory factor
Distribution of allele and genotype frequencies between various psoriatic subcategories
No significant differences were observed in the distribution of alleles and genotypes of the MIF-173*G/C polymorphism and MIF-794*CATT tetra-nucleotide repeats number variation in male versus female patients; in early versus late onset disease subjects; mild-to-moderate versus severe disease; in patients with short, intermediate, or longer duration of disease and in patients with or without any disease-associated comorbidities (p>0.05) [Table 4].
| Clinical variable | MIF-794 CATT genotypes (n = 265) | P | ||||||
|---|---|---|---|---|---|---|---|---|
| 5/5 (n = 20) | 6/6 (n = 101) | 7/7 (n = 3) | 5/6 (n = 87) | 5/7 (n = 13) | 6/7 (n = 40) | 6/8 (n = 1) | ||
| Age at onset (years) (mean, range) | 40 ± 15, 18–73 | 31.4 ± 15.7, 1–69 | 34 ± 24, 15–61 | 33.45 ± 14, 1–65 | 28 ± 16, 8–63 | 31 ± 17.3, 1–71 | 0.125 | |
| Duration of disease (years) (mean, range) | 7.5 ± 4.8, 0.5–20 | 8.8 ± 7, 0.25–40 | 7 ± 3, 4–10 | 8 ± 7, 0.08–34 | 12.1 ± 6.2, 3–26 | 8.7 ± 7.5, 0.50–35 | 0.290 | |
| PASI scores (mean) | 5.3 ± 4.2 | 6.1 ± 7.2 | 6.5 ± 8.2 | 5.8 ± 4.1 | 3.4 ± 1.8 | 7.1 ± 8.5 | 0.422 | |
| Comorbidities (n = 70; 26.4%), n(%) | 5 (7.1) | 23 (32.9) | 2 (2.9) | 22 (31.4) | 6 (8.6) | 11 (15.7) | 1 (1.4) | 0.881 |
| Serum MIF values* (mean, range) | 13.2 ± 5.3 | 14.3 ± 6.6 | - | 12.4 ± 5.6 | 16.7 ± 4.9 | 13.4 ± 6.5 | - | 1.000 |
*Serum MIF values were available in 100/265 patients. MIF: Migration inhibitory factor, PASI: Psoriasis area severity index
Serum migration inhibitory factor concentration
The mean serum MIF levels were significantly higher in patients (15.68±5.86 ng/ml vs. 7.75±2.72 ng/ml; P < 0.001). In receiver-operator characteristics curve analysis, the cutoff value was 9.8 ng/ml with a sensitivity and specificity of 88% and 76%, respectively [Figure 2]. No statistically significant correlation was found between the mean serum MIF levels and gender (P = 0.297), age of onset (P = 0.192) [Figure 3a], disease severity (P = 0.735) [Figure 3b], and presence or absence of comorbidities (P = 0.879). Although mean serum MIF levels were found to be minimally elevated in males, in patients with severe psoriasis and with early-onset psoriasis, in -173C allele carriers (GC+CC genotypes; n=45), and in patients with extended (>5) CATT repeats, that is, 6/6+6/7+6/8+7/7 genotypes (n=45), none of the results were statistically significant.
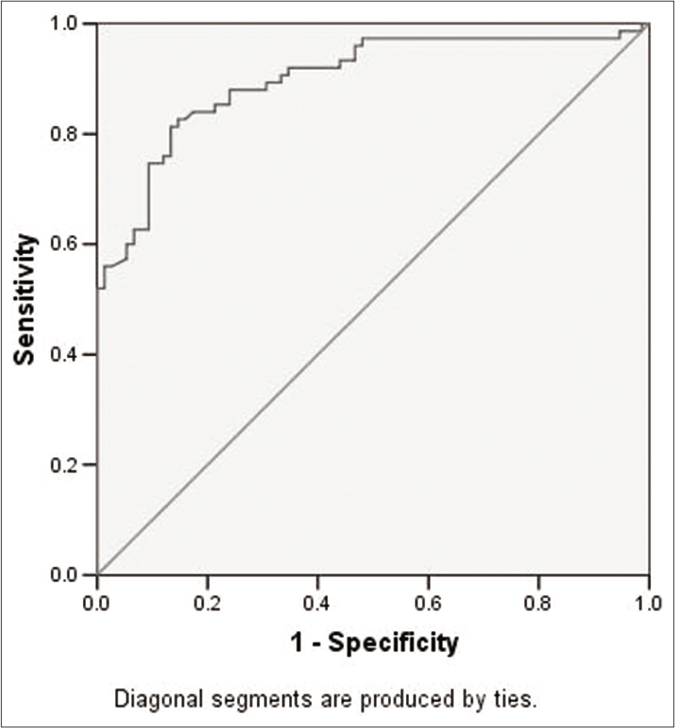
- Receiver-operator characteristics (ROC) curve analysis depicting area under the curve (0.899), sensitivity (88%) and specificity (76%) for serum MIF levels.
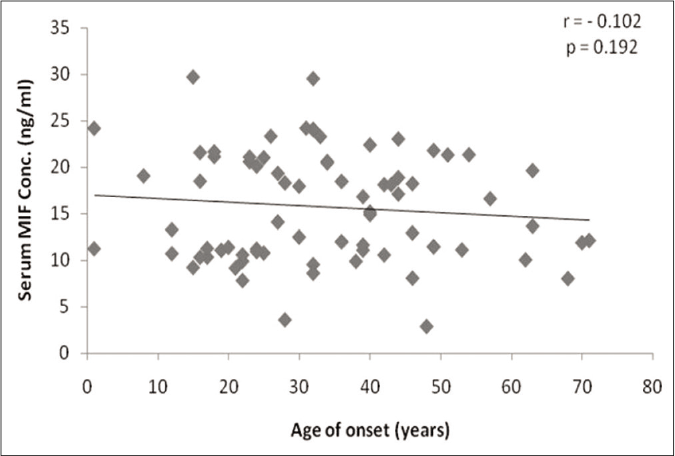
- Scatter plot with linear regression analysis showing correlation between serum MIF levels (ng/ml) and age of onset of chronic plaque psoriasis
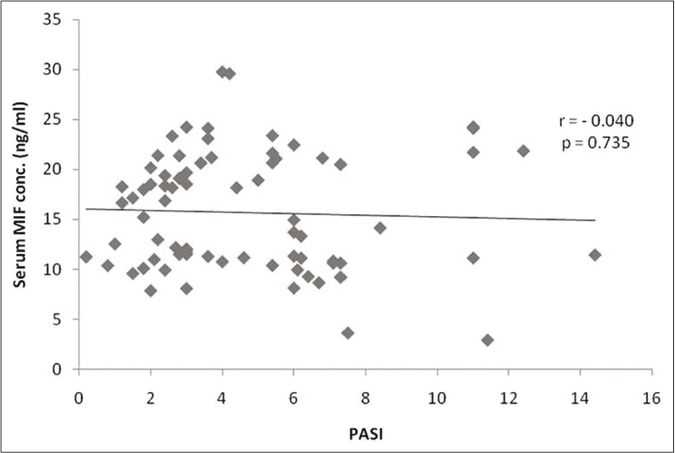
- Scatter plot with linear regression analysis showing correlation between serum MIF levels (ng/ml) and clinical severity of psoriasis in terms of PASI scores.
Discussion
The severity and course of illness differ across psoriatic patients and so does its management. Hence, it is important to classify patients into groups who might require aggressive and longer courses of therapy and those who can be managed more conservatively. The age of onset is one such marker which defines two subpopulations of patients. Early-onset (type I) psoriasis typically presents before the age of 40 years, with a peak onset at 16–22 years; approximately 70% of all psoriasis patients fall in this category. Late-onset (type II) psoriasis usually presents after 40 years, with a peak onset between 57–60 years.21,22 The results of this Indian cohort corroborate these findings as 69.8% of our patients had early-onset psoriasis. Early-onset psoriasis shows a strong association with Class I human leukocyte antigen (HLA) alleles, specifically HLA-C*06, whereas late-onset psoriasis is more sporadic, has an unclear genetic background, and evinces a greater association with disease associated comorbidities.22-25 In this study also, we found that the presence of comorbidities was more frequently noted in patients with late-onset disease (P = 0.01).
MIF is a pivotal regulator of innate immunity that promotes pro-inflammatory activities of various immune cells.26,27 Promoter polymorphisms in MIF gene influence basal as well as disease-associated MIF expression and its serum levels, which in turn have been shown to have enormous effects on disease phenotype in experimental models.28,29
The choice of single-nucleotide polymorphisms tested in the present study was principally based on earlier reports.9-12,14,15,18,28 Baugh et al. identified a functionally significant polymorphism in the human MIF gene, consisting of CATT repeats at position -794 of the MIF promoter.15 The 5-CATT repeat display lowers promoter activity when compared to the 6-, 7-, or 8 repeat alleles. Another polymorphism in the MIF promoter region is -173*G/C. In all populations studied, the -173*G allele (75%–90%) has been found to be far more common than the -173*C allele (15%–20%). MIF-173*C allele introduces activating enhancer binding protein 4 (AP-4) transcription factor binding site which in turn affects MIF gene expression, increasing MIF production.16
The previous association studies investigating these polymorphisms in various diseases across a variety of populations have given discordant results.9-12,14-17 Thus, it appears that the role of MIF is highly variable, depending on the populations, disease, and cell-types being studied. The genotype and haplotype frequencies of MIF polymorphisms in controls were found to be consistent with similar published studies from other South East Asian countries as already observed in our earlier study (unpublished data, in communication).10-12,16-18
This is the first report on the frequencies of these polymorphisms in North Indian psoriatic patients. In this study, 74% patients carried extended MIF -794*CATT repeats (>5, i.e., 6, 7, 8) which is expected to be associated with increased pro-inflammatory activity. Furthermore, the CATT5-MIF-173*C haplotype conferred an odds ratio of 3.03 for the risk of susceptibility to psoriasis. Donn et al. found that MIF-CATT7-173*C haplotype was important in susceptibility to psoriasis in a Caucasian population while Wu et al. did not observe any significant differences in the distribution of alleles, genotypes or haplotypes between patients and controls in the Han Chinese population.17,18 These differences in haplotype frequencies may be due to ethnic or genetic differences in, or environmental influences on, psoriasis among the populations analyzed.
Mallon et al.30 and Burden et al.31 observed an association between gender and psoriasis susceptibility. Wu et al. suggested a preliminary association between the MIF-173*C allele and male psoriasis and late-onset psoriasis in Han population from China.30,31 However, we did not find any such correlation.
We found that the serum MIF levels in patients were significantly elevated (P < 0.001), a finding similar to that of Shimizu et al.32 Shimizu et al reported more severe disease in their cohort of 48 patients with a mean psoriasis area severity index of 17.9±1.4 (range 3.2–41.4), and noted that severity of disease showed a linear correlation with serum MIF levels, which was not observed by us.32 Most patients in our cohort had mild-to-moderate disease severity (mean psoriasis area severity index score 5.45±4.63), with only 13 patients having psoriasis area severity index score>10. We suggest that it would be an oversimplification to expect a linear correlation between serum MIF levels and disease severity, since there might be numerous other factors that determine the latter. In addition, our results reiterate the effect of genetic heterogeneity among different ethnic populations on the pathogenesis of psoriasis.
Limitations
There are some limitations to this analysis. First, serum MIF levels were not measured in all patients, and second the lesional expression of MIF could not be studied due to financial constraints.
We attempted to evaluate genetic susceptibility in patients with chronic plaque psoriasis, using the candidate gene approach. We observed that MIF promoter variants have an impact on susceptibility to psoriasis. Association of CATT5-MIF-173*C haplotype with chronic plaque psoriasis was found to be significant and could be used as a genetic marker to identify individuals at high risk for developing psoriasis. This is a preliminary attempt to identify the phenotype of disease susceptible groups and it must be stressed that our results need to be validated in larger population samples.
Acknowledgments
This work is supported by a grant from the Fund for Science and Engineering Research (FSER): Science and Engineering Research Board (SERB), Department of Science and Technology (SB/FT/LS-176/2012 dated 26-04-2013).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Fund for Science and Engineering Research (FSER): Science and Engineering Research Board (SERB), Department of Science and Technology: SB/FT/LS-176/2012 dated 26-04-2013.
References
- Psoriasis in India: Prevalence and patterns. Indian J Dermatol Venereol Leprol. 2010;76:595-601.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SPP) Acta DermVenereol. 1997;77:273-6.
- [Google Scholar]
- Localization of macrophage migration inhibitory factor (MIF) to secretory granules within the corticotrophic and thyrotrophic cells of the pituitary gland. Mol Med. 1995;1:781-8.
- [CrossRef] [PubMed] [Google Scholar]
- An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996;183:277-82.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor in rheumatoid arthritis: Evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999;42:1601-8.
- [CrossRef] [Google Scholar]
- Macrophage migration inhibitory factor: Gene polymorphisms and susceptibility to inflammatory diseases. Clin Infect Dis. 2005;41(Suppl 7):S513-9.
- [CrossRef] [PubMed] [Google Scholar]
- Localization of the human gene for macrophage migration inhibitory factor (MIF) to chromosome 22q11.2. Genomics. 1997;39:235-6.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic cloning of mouse MIF (macrophage inhibitory factor) and genetic mapping of the human and mouse expressed gene and nine mouse pseudogenes. Genomics. 1995;27:405-11.
- [CrossRef] [PubMed] [Google Scholar]
- A novel 5'-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2001;44:1782-5.
- [CrossRef] [Google Scholar]
- Mutation screening of the macrophage migration inhibitory factor gene: Positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of MIF gene promoter variations on risk of rheumatic heart disease and its age of onset in Saudi Arabian patients. Front Immunol. 2016;7:98.
- [CrossRef] [PubMed] [Google Scholar]
- Functional polymorphisms in the promoter region of macrophage migration inhibitory factor and atopy. Am J Respir Crit Care Med. 2004;169:1014-8.
- [CrossRef] [PubMed] [Google Scholar]
- Complex genetic predisposition in adult and juvenile rheumatoid arthritis. BMC Genet. 2004;5:2.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor gene polymorphism is associated with sarcoidosis in biopsy proven erythema nodosum. J Rheumatol. 2002;29:1671-3.
- [Google Scholar]
- A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170-6.
- [CrossRef] [PubMed] [Google Scholar]
- Variations MIF gene polymorphisms in psoriasis in ncRNA gene LOC284889 and MIF-794CATT repeats are associated with malaria susceptibility in Indian populations. Malar J. 2013;12:345.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol. 2004;123:484-7.
- [CrossRef] [PubMed] [Google Scholar]
- Association of MIF promoter polymorphisms with psoriasis in a Han population in northeastern China. J Dermatol Sci. 2009;53:212-5.
- [CrossRef] [PubMed] [Google Scholar]
- New immune system genetic polymorphisms associated with moderate-to-severe plaque psoriasis: A case-control study. Br J Dermatol. 2015;172:1432-5.
- [CrossRef] [PubMed] [Google Scholar]
- Association analysis of GWAS and candidate gene loci in a Pakistani population with psoriasis. Mol Immunol. 2015;64:190-4.
- [CrossRef] [PubMed] [Google Scholar]
- Age at disease onset: A key factor for understanding psoriatic disease. Rheumatology (Oxford). 2014;53:1178-85.
- [CrossRef] [PubMed] [Google Scholar]
- The major psoriasis susceptibility locus PSORS1 is not a risk factor for late-onset psoriasis. J Invest Dermatol. 2005;124:103-6.
- [CrossRef] [PubMed] [Google Scholar]
- Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients-an analysis of 1019 HLA-Cand HLA-B-typed patients. J Invest Dermatol. 2006;126:740-5.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis and type 2 diabetes risk among psoriatic patients in a Spanish population. Australas J Dermatol. 2012;53:128-30.
- [CrossRef] [PubMed] [Google Scholar]
- MIF, MIF alleles, and prospects for therapeutic intervention in autoimmunity. J Clin Immunol. 2013;33(Suppl 1):S72-8.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor gene polymorphisms in inflammatory bowel disease: An association study in New Zealand Caucasians and meta-analysis. World J Gastroenterol. 2013;19:6656-64.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 2005;52:3020-9.
- [CrossRef] [PubMed] [Google Scholar]
- Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256-70.
- [CrossRef] [PubMed] [Google Scholar]
- HLA-CW*0602 is a susceptibility factor in type I psoriasis, and evidence Ala-73 is increased in male type I psoriatics. J Invest Dermatol. 1997;109:183-6.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics of psoriasis: Paternal inheritance and a locus on chromosome 6p. J Invest Dermatol. 1998;110:958-60.
- [CrossRef] [PubMed] [Google Scholar]
- High macrophage migration inhibitory factor (MIF) serum levels associated with extended psoriasis. J Invest Dermatol. 2001;116:989-90.
- [CrossRef] [PubMed] [Google Scholar]






