Translate this page into:
Skin substitutes in dermatology
Correspondence Address:
Sudha Anish
107, 919 West 13 Avenue, Vancouver, British Columbia, V5Z1P4
Canada
| How to cite this article: Anish S. Skin substitutes in dermatology. Indian J Dermatol Venereol Leprol 2015;81:175-178 |
INTRODUCTION
Skin substitutes are a heterogeneous group of biological and/or synthetic elements that facilitate wound closure and replace the functions of skin, either temporarily or permanently. [1] The history of skin substitutes dates back to as early as 1500 BC when xenografts were used for wound coverage. [2] Xenografts gave way to homografts such as cadaveric skin, amnion, and autografts. Newer technologies paved the way for bioengineered skin substitutes. [1],[2]
No perfect or ideal skin substitute exists. An ideal skin substitute is non-toxic, immunologically compatible, has low antigenicity, and does not transmit disease. The skin substitutes function to minimize the loss of water, electrolytes, and protein, reduce bacterial load provide coverage of tendons, nerves, and vessels thus preventing desiccation, decrease pain, restore function, and facilitate early movement. [3]
Skin substitutes can be classified into three types [Table - 1] according to:
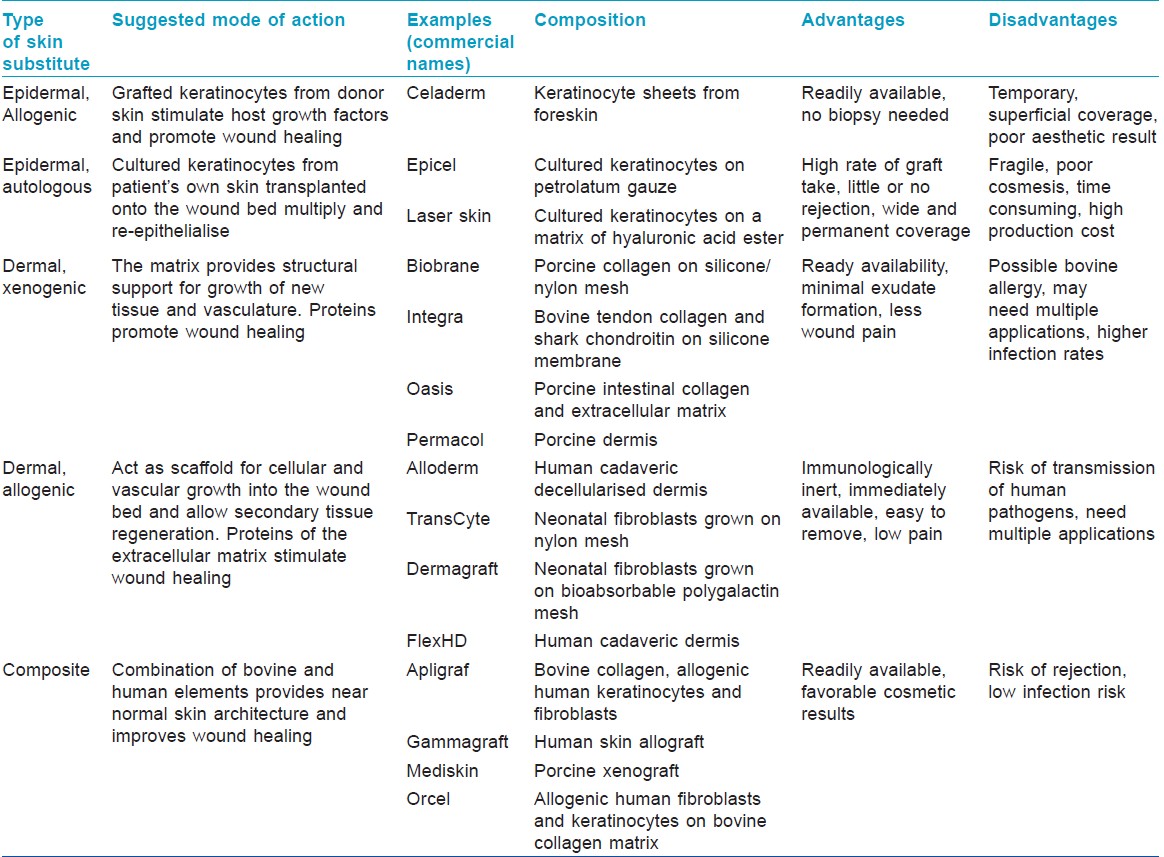
- Skin layer to be replaced: Subdivided into epidermal, dermal, dermal-epidermal composites
- Durability: Temporary and permanent
- Origin of grafting material: Biologic - those generated from biologic materials such as animal or human tissue (allogenic, autogenic, or xenogenic); synthetic - produced in the laboratory; biosynthetic - combination of synthetic and biologic elements. [4],[5]
Several skin substitutes are currently available for a variety of applications. Naturally occurring or biological materials like amnion, cadaveric skin allograft and porcine skin xenografts are used worldwide as temporary skin substitutes. [2] Alloderm, TransCyte, Kollagen, and NeuSkin are some of the commercially available products in India. The choice of a suitable substitute for each clinical application depends upon their advantages and disadvantages.
A. Clinical Applications
Burns
Skin substitutes can play a major role in the treatment of burns as they aid in restoration of cutaneous continuity. [1] Various types of skin substitutes have been studied and proven to be useful in the management of partial and full thickness burns. [6],[7] They are effective, improve wound healing, and decrease the duration of hospitalization. [6],[8],[9],[10],[11],[12]
Ulcers resistant to conventional healing
The healing success of any chronic wound depends essentially on its wound bed. [13] Skin substitutes not only provide a covering for the ulcer, but also actively participate in the healing process by stimulating angiogenesis and reepithelialisation. [14] Numerous randomized controlled studies have assessed the efficacy of various skin substitutes and have been proven to improve wound healing in venous ulcers, diabetic foot ulcers and pressure ulcers. [15],[16],[17],[18],[19],[20]
In a large multicentre randomized study, a composite graft (Apligraf) was found to be significantly better in healing large venous ulcers of more than one year duration than compression therapy alone. [15] Marston et al. in a large multicenter randomized controlled prospective study found that wounds treated with a dermal allogenic graft (Dermagraft) healed significantly faster than conventionally treated wounds. [17] In a study on 23 patients, Brem et al. found that 13 of the 21 pressure ulcers treated with a composite graft (Apligraf) healed in a mean time of 29 days. [20]
Cutaneous repair following surgery for skin cancer
Many studies report the usefulness of skin substitutes in repair of wounds following excision of cutaneous malignancies. [21],[22]
Dermatologic conditions
Pyoderma gangrenosum
The etiology of the condition is still unclear. Conventional treatment includes corticosteroids and immunosuppressants. Treatment with cultured human skin equivalent (Graftskin) in a 26-year-old female showed 30-40% wound closure rate in the first 2 weeks, with complete reepithelialisation at the end of 6 weeks. [23] There have also been case reports with other skin substitutes, showing favorable responses in pyoderma gangrenosum. [24],[25],[26],[27],[28] However, controlled studies are required to confirm these observations.
Vitiligo
Vitiligo is a common depigmenting disorder with limited therapeutic possibilities. Therapeutic use of cultured epidermis and melanocytes has been promising in the treatment of vitiligo. [29] Andreassi et al. grafted autologous keratinocyte cultures in 11 vitiligo patients and demonstrated progressive improvement in the condition at 3, 6, 12, and 18 months achieving 90-100% repigmentation in 6 of them. [30] Other researchers have used melanocyte cultures with satisfactory results. [31],[32]
Other skin disorders
Skin substitutes have also been applied successfully in healing of wounds in cases of epidermolysis bullosa, [33] aplasia cutis, [34] harlequin ichthyosis, [35] ulcerative sarcoidosis, [36] necrobiosis lipoidica, [37] and bullous morphea. [38]
B. Laboratory Applications
Tissue engineered skin has been found useful in research studies involving various skin diseases. Reconstructed skin models have augmented research analysis involving the cellular and immunological elements of psoriasis, the study of skin pigmentation, skin melanoma, wound healing and allergens with greater flexibility, increased convenience, good reproducibility, and reduced costs. [39],[40],[41],[42] Genetic modification of cultured skin grafts can act as vehicles for cutaneous gene therapy in conditions such as epidermolysis bullosa [43] and Netherton syndrome. [44]
CHALLENGES
Despite the favorable results, skin substitutes cannot replace all the native functions of skin as the currently available ones contain at most only two skin components, thus influencing engraftment, aesthetic, and functional outcome. Wound bed preparation is a major challenge in case of skin substitutes requiring revascularization. Inadequate angiogenesis can lead to rejection of the skin substitute. It is also most vulnerable to infection at this stage. Hypopigmentation or uneven distribution of pigmentation may occur, either due to the absence of melanocytes or melanocyte retention. Compared with normal skin tissue, scars that develop at the margins of skin substitutes are less resistant to mechanical tension and have poorer function and aesthetic qualities. Transmission of infection is a major concern involving skin substitutes though meticulous precautions are taken during all stages of their preparation. [13],[45]
CONCLUSION
The development of a multitude of skin substitutes has expanded the options for dermatologic surgeons when treating complex wounds. Familiarity with their components, uses, strengths, and disadvantages could facilitate the appropriate use of these products for dermatologic conditions.
| 1. |
Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: A review. Adv Skin Wound Care 2007;20:493-508.
[Google Scholar]
|
| 2. |
Halim AS, Khoo TL, Mohd. Yussof SJ. Biologic and synthetic skin substitutes: An overview. Indian J Plast Surg 2010;43:S23-8.
[Google Scholar]
|
| 3. |
Wood BC. Skin grafts. Emedicine article (last updated on 2013 May 3). Available from: http://emedicine.medscape.com/article/1295109-overview. [Last accessed on 2014 June 25].
[Google Scholar]
|
| 4. |
Ferreira MC, Paggiaro AO, Isaac C, Neto NT, Santos GB. Skin substitutes: Current concepts and a new classification system. Rev Bras Cir Plast 2011;26:696-702.
[Google Scholar]
|
| 5. |
Cronin H, Goldstein G. Biologic skin substitutes and their applications in dermatology. Dermatol Surg 2013;39:30-4.
[Google Scholar]
|
| 6. |
Supp DM, Boyce ST. Engineered skin substitutes: Practices and potentials. Clin Dermatol 2005;23:403-12.
[Google Scholar]
|
| 7. |
Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: A systematic review. Burns 2007;33:946-57.
[Google Scholar]
|
| 8. |
Shakespeare PG. The role of skin substitutes in the treatment of burn injuries. Clin Dermatol 2005;23:413-8.
[Google Scholar]
|
| 9. |
Hartford CE, Wang XW, Peterson VM, Rodgers CM, Ketch LL. Healing characteristics of expanded autografts on wound covered with homograft and biobrane temporary wound dressing. J Burn Care Rehabil 1989;10:476-80.
[Google Scholar]
|
| 10. |
Waymack P, Duff RG, Sabolinski M. The effect of a tissue engineered bilayered living skin analogue, over meshed split-thickness autografts on the healing of excised burn wounds. The Apligraf Burn Study Group. Burns 2000;26:609-19.
[Google Scholar]
|
| 11. |
Burd A, Chiu T. Allogenic skin in the treatment of burns. Clin Dermatol 2005;23:376-87.
[Google Scholar]
|
| 12. |
Lal S, Barrow RE, Wolf SE, Chinkes DL, Hart DW, Heggers JP, et al. Biobrane improves wound healing in burned children without increased risk of infection. Shock 2000;14:314-9.
[Google Scholar]
|
| 13. |
Greaves NS, Iqbal SA, Baguneid M, Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Rep Reg 2013;21:194-210.
[Google Scholar]
|
| 14. |
Han G. State- of- the- art wound healing: Skin substitutes for chronic wounds. Cutis 2014;93:E13-6.
[Google Scholar]
|
| 15. |
Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogenic cultured human skin equivalent. Human skin Equivalent Investigators Group. Arch Dermatol 1998;134:293-300.
[Google Scholar]
|
| 16. |
Edmonds M. European and Australian Apligraf Diabetic Foot ulcer Study Group. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds 2009;8:11-8.
[Google Scholar]
|
| 17. |
Marston WA, Hanft J, Norwood P, Pollack R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomised trial. Diabetes Care 2003;26:1701-5.
[Google Scholar]
|
| 18. |
Romanelli M, Dini V, Bertone MS. Randomised comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Adv Skin Wound Care 2010;23:34-8.
[Google Scholar]
|
| 19. |
Gibbs S, Van den Hoogenband HM, Kirtschig G, Richters CD, Spiekstra SW, Breetveld M, et al. Autologous full thickness skin substitute for healing chronic wounds. Br J Dermatol 2006;155:267-74.
[Google Scholar]
|
| 20. |
Brem H, Balledux J, Bloom T, Kerstein MD, Hollier L. Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: A new paradigm in wound healing. Arch Surg 2000;135:627-34.
[Google Scholar]
|
| 21. |
Gohari S, Gambla C, Healey M, Spaulding G, Gordon KB, Swan J, et al. Evaluation of tissue- engineered skin (human skin substitute) and secondary intention healing in the treatment of full thickness wounds after Mohs micrographic or excisional surgery. Dermatol Surg 2002;28:1107-14.
[Google Scholar]
|
| 22. |
Gath HJ, Hell B, Zarrinbal R, Bier J, Raguse JD. Regeneration of intraoral defects after tumour resection with a bioengineered human dermal replacement (Dermagraft). Plast Reconstruct Surg 2002;109:889-93.
[Google Scholar]
|
| 23. |
De Imus G, Golomb C, Wilkel C, Tsoukas M, Nowak M, Falanga V. Accelerated healing of pyoderma gangrenosum treated with bioengineered skin and concomitant immunosuppression. J Am Acad Dermatol 2001;44:61-6.
[Google Scholar]
|
| 24. |
Limova M, Mauro T. Treatment of pyoderma gangrenosum with cultured keratinocyte autografts. J Dermatol Surg Oncol 1994;20:833-6.
[Google Scholar]
|
| 25. |
Long RE, Falabella AF, Valencia I, Eaglstein WH, Kirsner RS. Treatment of refractory, atypical lower extremity ulcers with tissue engineered skin (Apligraf). Arch Dermatol 2001;137:1660-61.
[Google Scholar]
|
| 26. |
Philips TJ. Bigby M, Bercovitch L. Cultured allografts as an adjunct to the medical treatment of problematic leg ulcers. Arch Dermatol 1991;127:799-801.
[Google Scholar]
|
| 27. |
Toyozawa S, Yamamoto Y, Nishide T, Kishioka A, Kanazawa N, Matsumoto Y, et al. Case report: A case of pyoderma gangrenosum with intractable leg ulcers treated by allogenic cultured dermal substitutes. Dermatol Online J 2008;14:17. Available from: http://escholarship.org/uc/item/1bs0h111. [Last accessed on 2014 June 25.
[Google Scholar]
|
| 28. |
Dean SJ, Nieber S, Hickerson WL. The use of cultured epithelial autograft in a patient with idiopathic pyoderma gangrenosum. Ann Plast Surg 1991;26:194-5.
[Google Scholar]
|
| 29. |
Pianigiani E, Andreassi A, Andreassi L. Autografts and cultured epidermis in the treatment of vitiligo. Clin Dermatol 2005;23:424-9.
[Google Scholar]
|
| 30. |
Andreassi L, Pianigiani E, Andreassi A, Taddeucci P, Biagioli M. A new model of epidermal culture for the surgical treatment of vitiligo. Int J Dermatol 1998;37:595-8.
[Google Scholar]
|
| 31. |
Chen YF, Yang PY, Hung CM, Hu DN. Transplantation of autologous cultured melanocytes for treatment of large segmental vitiligo. J Am Acad Dermatol 2001;44:543-5.
[Google Scholar]
|
| 32. |
Olsson MJ, Juhlin L. Repigmentation of vitiligo by transplantation of cultured autologous melanocytes. Acta Derm Venereol 1993;73:49-51.
[Google Scholar]
|
| 33. |
Fivenson DP, Scherschun L, Choucair M, KuKuruga D, Young J, Shwayder T. Graftskin therapy in epidermolysis bullosa. J Am Acad Dermatol 2003;48:886-92.
[Google Scholar]
|
| 34. |
Bui D, Ikeda C. Reconstruction of aplasia cutis congenita (group V) of the trunk in a newborn. Plast Reconstr Surg 2003;111:2119-220.
[Google Scholar]
|
| 35. |
Culican SM, Custer PL. Repair of cicatrical ectropion in an infant with harlequin ichthyosis using engineered human skin. Am J Ophthalmol 2002;134:442-3.
[Google Scholar]
|
| 36. |
Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with Apligraf. Dermatology 2001;202:367-70.
[Google Scholar]
|
| 37. |
Owen CM, Murphy H, Yates VM. Tissue engineered dermal skin grafting in the treatment of ulcerated necrobiosis lipoidica. Clin Exp Dermatol 2001;26:176-8.
[Google Scholar]
|
| 38. |
Martin LK, Kirsner RS. Ulcers caused by bullous morphea treated with tissue engineered skin. Int J Dermatol 2003;42:402-4.
[Google Scholar]
|
| 39. |
Guerrero-Aspizua S, Garcia M, Murillas R, Retamosa L, Illera N, Duarte B, et al. Development of a bioengineered skin humanised mouse model for psoriasis: Dissecting epidermal-lymphocyte interacting pathways. Am J Pathol 2010;177:3112-24.
[Google Scholar]
|
| 40. |
Berking C, Herlyn M. Human skin reconstruct models: A new application for studies of melanocyte and melanoma biology. Histol Histopathol 2001;16:669-74.
[Google Scholar]
|
| 41. |
Martinez- Santamaria L, Guerrero- Aspizua S, Del Rio M. Skin bioengineering: Preclinical and clinical applications. Actas Dermosifiliogr 2012;103:5-11.
[Google Scholar]
|
| 42. |
Tornier C, Rosdy M, Maibach HI. In vitro skin irritation testing on reconstituted human epidermis: Reproducibility for 50 chemicals tested with two protocols. Toxicol In Vitro 2006;20:401-16.
[Google Scholar]
|
| 43. |
Gache Y, Baldeschi C, Del Rio M, Gagnoux- Palacios L, Larcher F, Lacour JP, et al. Construction of skin equivalents for gene therapy of recessive dystrophic epidermolysis bullosa. Hum Gene Ther 2004;15:921-33.
[Google Scholar]
|
| 44. |
Di WL, Larcher F, Semenova E, Talbot GE, Harper JI, Del Rio M, et al. Ex vivo gene therapy restores LEKTI activity and the architecture of Netherton syndrome derived skin grafts. Mol Ther 2011;19:408-16.
[Google Scholar]
|
| 45. |
Kamel RA, Ong JF, Eriksson E, Junker JP, Caterson EJ. Tissue engineering of skin. J Am Coll Surg 2013;217:533-55.
[Google Scholar]
|
Fulltext Views
10,026
PDF downloads
4,853





