Translate this page into:
Sorafenib-induced grade III hand–foot skin reaction with ulcerative dermatitis on scrotum, penis, and earlobe
Correspondence Address:
Kananbala Sahu
Department of Dermatology, All India Institute of Medical Sciences, Bhubaneswar, Odisha
India
| How to cite this article: Sirka CS, Sahu K, Pradhan S, Rout AN. Sorafenib-induced grade III hand–foot skin reaction with ulcerative dermatitis on scrotum, penis, and earlobe. Indian J Dermatol Venereol Leprol 2019;85:623-626 |
Sir,
A 60-year-old male with grade III hepatocellular carcinoma taking sorafenib 400 mg twice daily since 10 days was referred to the dermatology outpatient department for erosions and ulcerations on scrotum, penis, and frictional zones like sacral areas and buttock. On dermatological examination, there were symmetrical erythematous patches with surface ulceration over groin, scrotum, penis, and gluteal region [Figure - 1]a and [Figure - 1]b. In addition, erythematous plaques with central crusting and necrosis over bilateral earlobes were noted[Figure - 1]c and [Figure - 1]d. On the background of decreased food intake due to hepatocellular carcinoma and clinical features, acquired zinc deficiency and sorafenib-induced hand–foot skin reaction were suspected. However, during hospital stay he developed new erythematous patches with characteristic perilesional halo over palmar aspect of all finger tips, toes, and heel [Figure - 1]e and [Figure - 1]f. Few of these patches had central blistering and few were hyperkeratotic. All routine investigations were within normal limit except liver enzymes [total S. bilirubin 1.3 mg/dL, aspartate transaminase 194 U/L (0–37 U/L), alanine transaminase 80 U/L (0–40 U/L), alkaline phosphatase 800 U/L (100–290 U/L)]. His serum zinc level was 100 μg/dL (90–150 μg/dL). Histopathology from the erythematous patch revealed hyperkeratosis, focal parakeratosis, and irregular acanthosis with subcorneal bulla filled with neutrophils and fibrin. Papillary dermis showed mild lymphocytic infiltration [Figure - 2]. Hence, final diagnosis of sorafenib-induced hand–foot skin reaction was made and the drug was discontinued. After 2 weeks, the skin lesions improved completely [Figure - 3]. He was issued a drug card and capecitabine was started for hepatocellular carcinoma. On subsequent follow-up, the patient did not develop any similar skin lesions.
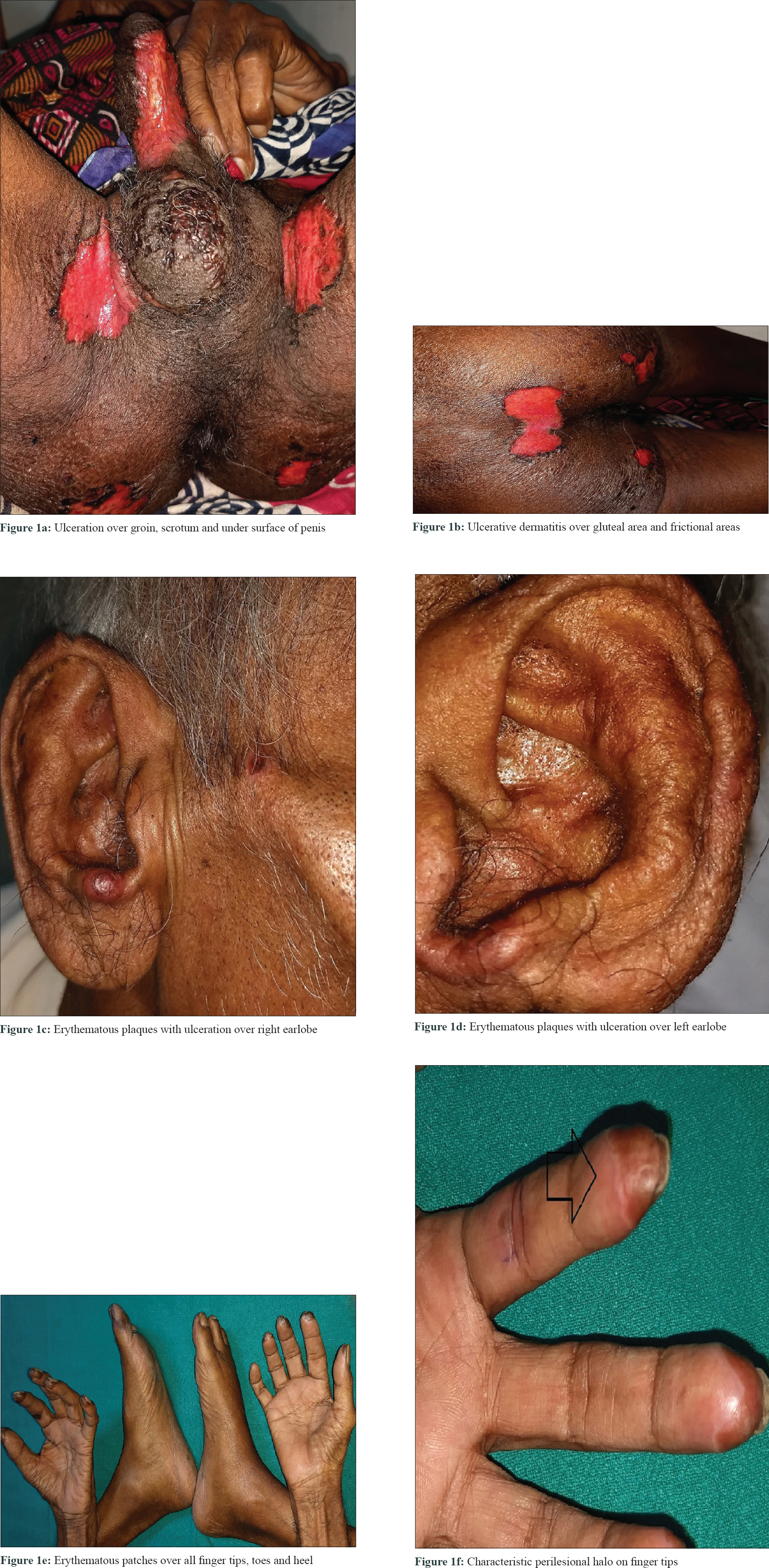 |
| Figure 1: |
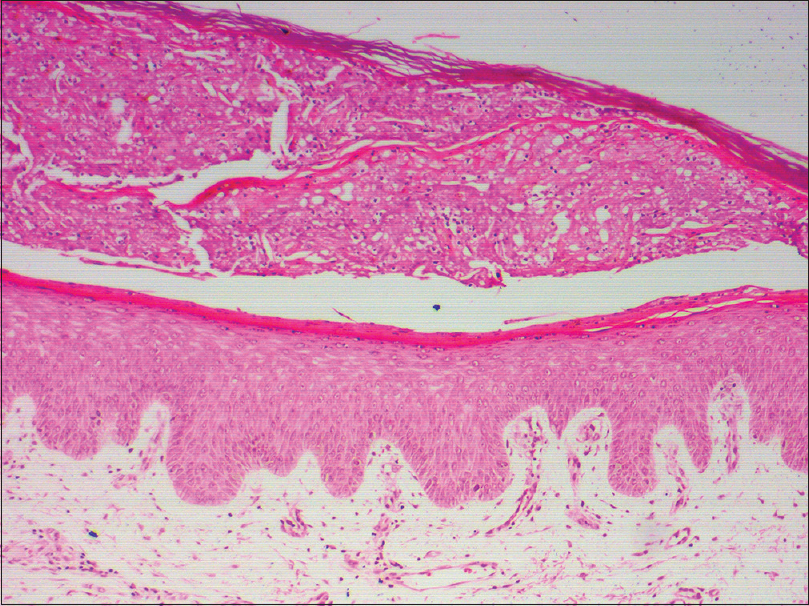 |
| Figure 2: Hyperkeratosis, focal parakeratosis, irregular acanthosis with subcorneal bulla filled with neutrophils and fibrin (H and E, �100) |
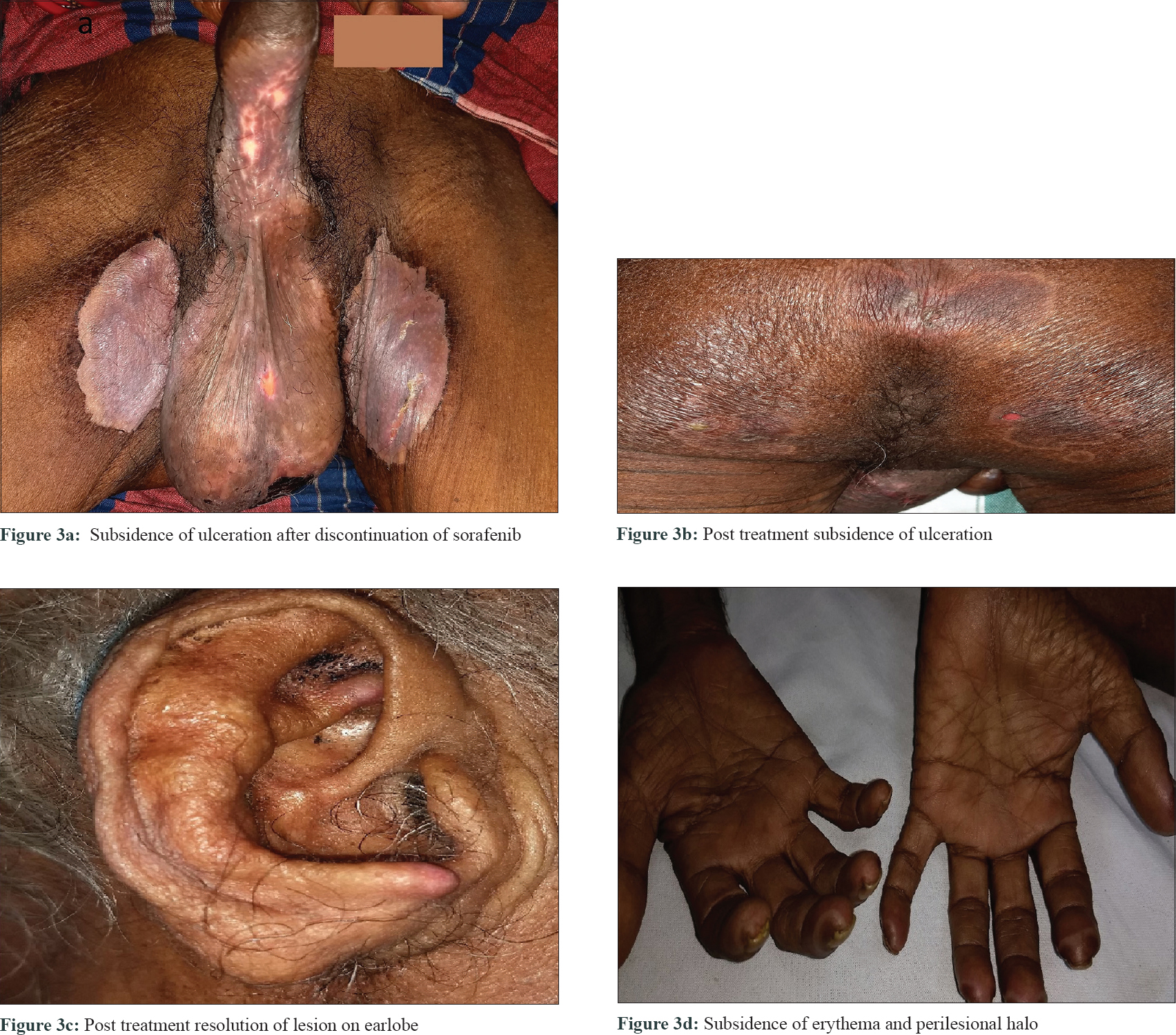 |
| Figure 3: |
Sorafenib is a multikinase inhibitor, which has been recently approved for the treatment of carcinomas such as hepatocellular carcinoma.[1] Dermatological adverse events associated with sorafenib include hand–foot skin reaction, facial erythema (rash and desquamation), splinter subungual hemorrhage, alopecia, pruritus, and xerosis.[2] The prevalence of hand–foot skin reaction is 10%–62% in patients treated with sorafenib.[3] Clinically, hand–foot skin reaction develops within the first 2–6 weeks of treatment as hyperkeratotic lesions, with the formation of calluses and superficial blisters on an erythematous base, mainly affecting the palms and soles bilaterally. The lesions usually have a characteristic peripheral erythematous halo. Symptoms are dose-related and are noted in areas of skin subject to pressure or friction.[3] We found only one previous case report of sorafenib-induced hand–foot skin reaction with scrotal lesion.[4] The National Cancer Institute has classified the lesions into three grades according to their severity. Minimal skin changes without pain (grade I), skin changes or pain, not interfering with function (grade II), and ulcerative dermatitis or skin changes with pain interfering with function (grade III). The majority of the cases are reported to be mild to moderate (grades I and II), responding to topical treatment with high-potency corticosteroids. Severe involvement, as in our patient, is less frequent and usually necessitates sorafenib discontinuation. The pathogenesis of acral erythema is still unknown, but the dose dependence in most cases suggests a direct toxic effect. Another proposed mechanism is that subclinical trauma may result in leakage of the drug through capillaries and may inhibit vascular endothelial growth factor receptors and platelet-derived growth factor receptor–mediated capillary repair and regeneration, interfering with tissue regeneration. This hypothesis is supported by the appearance of lesions over sites of friction.[5] The diagnosis of this reaction is primarily based on temporal correlation of drug intake and clinical suspicion with lesions on the palms and soles. Histological examination is not necessary for diagnosis and shows epidermal parakeratosis and dyskeratosis with band-like areas of necrotic keratinocytes along with superficial bullous lesions. The treatment is mainly symptomatic for mild disease and a severe episode can require dose reduction, the temporary interruption of treatment, or even permanent withdrawal of the drug. In our case, the patient developed initially ulcerative dermatitis over frictional areas and earlobe instead of classical lesions of hand–foot skin reaction (erythematous patches with blistering and perilesional halo) over finger tips and sole. Although there is a single report of scrotal involvement associated with hand–foot skin reaction due to sorafenib, simultaneous presentation with ulcerative dermatitis over scrotum, penis, and earlobe has not been described previously in literature. Hence, the present case posed a diagnostic dilemma with acquired zinc deficiency at initial presentation until the appearance of classical lesions on finger tips. We believe the lesions on earlobes and scrotal skin is possibly due to induced trauma and capillary leakage of drug as proposed by Sibaud et al.[5] We report this case to emphasize the severe type of grade III hand–foot skin reaction due to a common chemotherapeutic drug (sorafenib) along with additional findings of ulcerative dermatitis over scrotum, penis, and earlobes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90.
[Google Scholar]
|
| 2. |
Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:2505-12.
[Google Scholar]
|
| 3. |
Lipworth AD, Robert C, Zhu A ×. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): Focus on sorafenib and sunitinib. Oncology 2009;77:257-71.
[Google Scholar]
|
| 4. |
Guerra JR, Suelves AM, Bella A, Lolo D. Hand, foot and scrotal blisters in a patient with cancer receiving oral chemotherapy. BMJ Case Rep 2014;2014. pii: bcr2013202822.
[Google Scholar]
|
| 5. |
Sibaud V, Delord JP, Chevreau C. Sorafenib-induced hand-foot skin reaction: A Koebner phenomenon? Target Oncol 2009;4:307-10.
[Google Scholar]
|
Fulltext Views
5,794
PDF downloads
1,711





