Translate this page into:
Spontaneous regression in an ulcerated CK7 positive Merkel cell carcinoma
2 Department of Pathology, Government Medical College, Kozhikode, Kerala, India
Correspondence Address:
Anza Khader
Department of Dermatology, 5/1986 B, "Maskan" Rajiv Nagar Colony, PO Puthiyara, 673004 Kozhikode, Kerala
India
| How to cite this article: Khader A, George S, Balakrishnan S, Aravindan KP. Spontaneous regression in an ulcerated CK7 positive Merkel cell carcinoma. Indian J Dermatol Venereol Leprol 2015;81:170-173 |
Abstract
Merkel cell carcinoma is an aggressive and frequently lethal tumor of the elderly, associated with sun exposure and immunosuppression which is less common in the dark-skinned. We report the case of a 40-year-old woman who presented with multiple slowly progressive, mildly itchy ulcerated plaques of size ranging from 2 × 3 cm to 5 × 7 cm on the left knee of 1 year duration. Skin biopsy showed diffuse dermal infiltration by small round cells with molding of cells and lymphocyte infiltration. The cells stained positive for cytokeratin (CK) 20, CK7, neuron-specific enolase, and chromogranin. The skin lesions underwent spontaneous regression within 1 month of skin biopsy and have not recurred during the past 2 years. The immune mechanisms triggered by biopsy possibly explain the spontaneous regression.INTRODUCTION
Merkel cell carcinoma (MCC) is an aggressive and frequently lethal tumor originating from the cutaneous Merkel cell, a neuroendocrine cell. It is a rare tumor of the elderly, which is less common in dark skin and is highly associated with sun exposure and immunosuppression. [1] Here, we report a case of Merkel cell carcinoma on a non-sun-exposed site in an immunocompetent dark-skinned patient, which showed ulceration and subsequent spontaneous regression.
Case Report
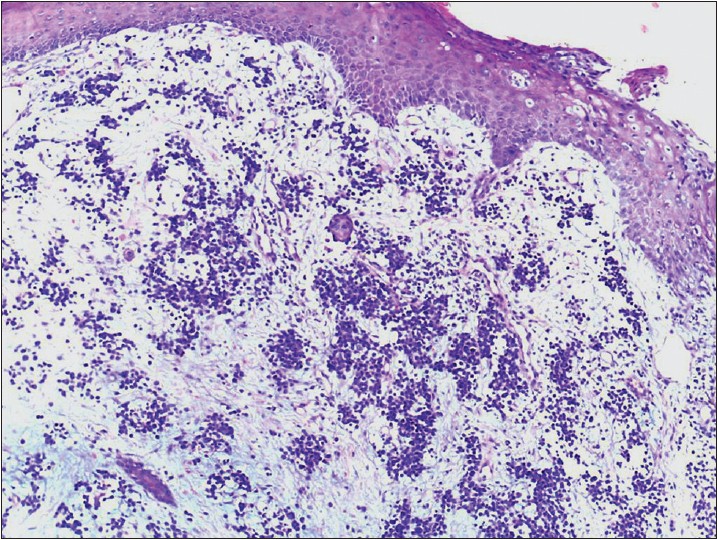
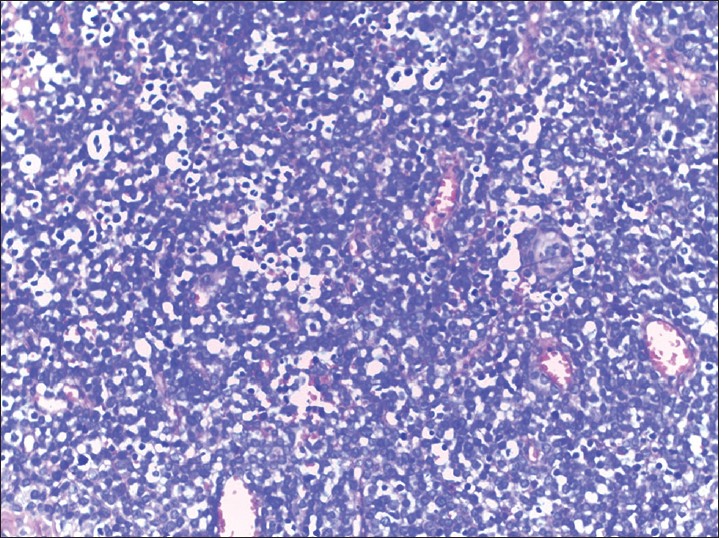
A 40-year-old woman presented with multiple raised, erythematous to hyperpigmented, ulcerated and crusted plaques ranging in size from 2 × 3 cm to 5 × 7 cm, arranged in an arciform pattern over the left knee [Figure - 1]. The lesions were mildly itchy and had slowly progressed over the previous 1 year. There was no lymphadenopathy and systemic examination was within normal limits. There was no history of excessive sun exposure, previous surgery or drug intake. The differential diagnoses considered included lupus vulgaris, primary diffuse large B-cell cutaneous lymphoma-leg type, and keloidal blastomycosis. Hematological examination, liver and renal function tests were normal. Serological tests for human immunodeficiency virus (HIV) 1 and 2 were negative. Mantoux test and tissue smear for acid-fast bacilli and fungus were negative. Skin biopsy showed diffuse dermal infiltration by small round cells with vesicular nuclei, indistinct nucleoli, occasional whorled structures due to moulding of cells (ball-in-mitt arrangement), atypical mitoses, and lymphocyte infiltration [Figure - 2], [Figure - 3], [Figure - 4]. The cells stained positive for cytokeratin (CK) 20, CK7, neuron-specific enolase (NSE), and chromogranin and negative for thyroid transcription factor-1 (TTF-1), CD45, and CD20, confirming the diagnosis of Merkel cell carcinoma [Figure - 5] and [Figure - 6]. Chest X-ray, abdominal and pelvic ultrasound, and computerized tomography of the thorax and abdomen were unremarkable.
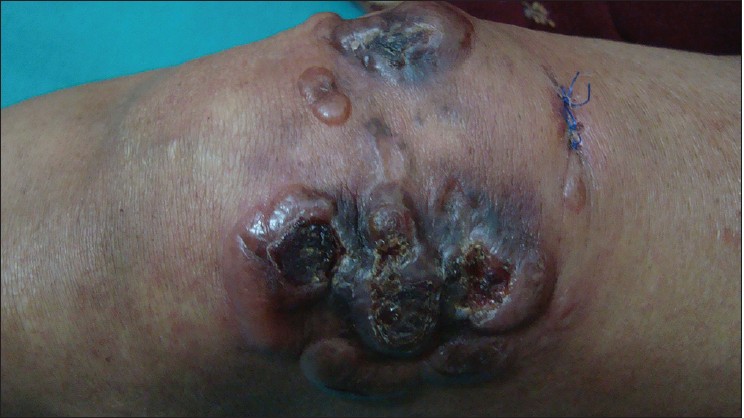 |
| Figure 1: Plaques with ulceration over the left knee joint |
 |
| Figure 2: Small round cells in the dermis (H and E, ×100) |
 |
| Figure 3: Starry sky appearance due to infiltrating lymphocytes (H and E, ×200) |
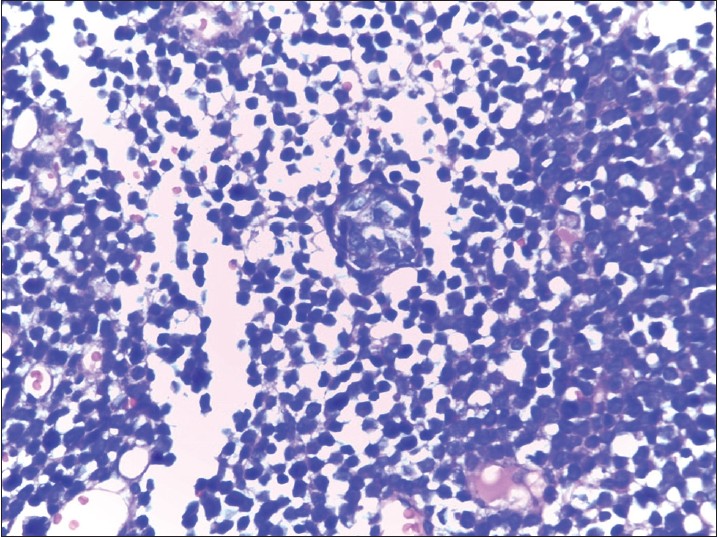 |
| Figure 4: Ball-in-mitt arrangement of the cells (H and E, ×400) |
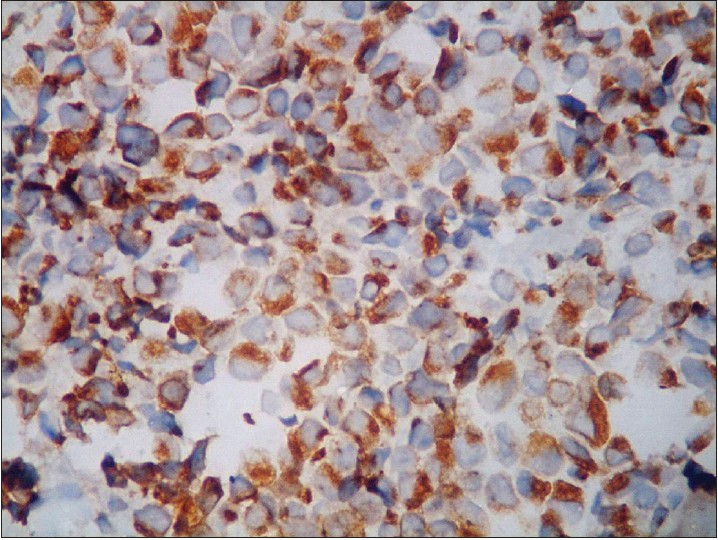 |
| Figure 5: Strongly positive CK20 (×400) |
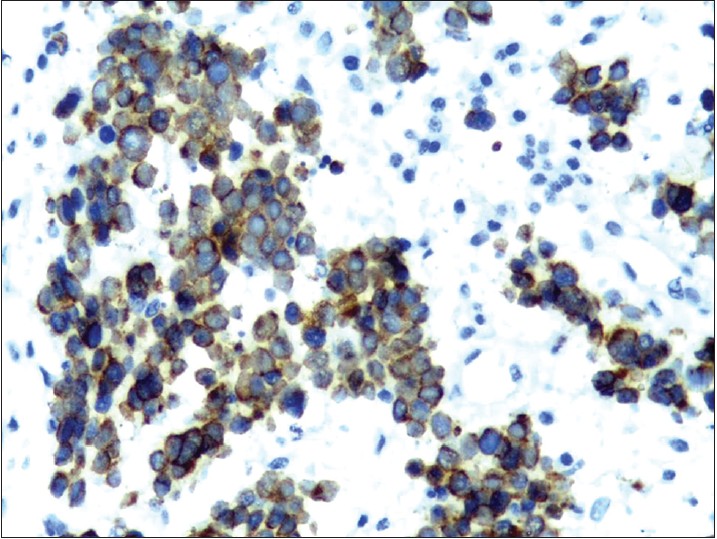 |
| Figure 6: Strongly positive CK7 (×400) |
The patient was referred to an oncologist for further management. However, the lesions underwent spontaneous regression within 1 month and no recurrence has been noticed during follow-up over the past 2 years.
DISCUSSION
Human Merkel cells were first described by Friedrich S. Merkel in 1875 who named them Tastzellen. [2] In 1972, Toker described five cases of what he called trabecular cell carcinoma of the skin and in 1980, De Wolff-Peeters et al. used the term Merkel cell tumor. [3],[4]
Mrerkel cell carcinoma is a rare malignancy appearing in the sixth to seventh decade of life, with a slight male preponderance. It affects predominantly the Caucasian race and is exceptionally rare in dark-skinned people. Sun exposure is one of the risk factors as C to T mutations induced by ultraviolet light contribute to its pathogenesis. Common sites of the tumor are head and neck, followed by the extremities and trunk. It has also been reported in HIV-infected individuals, immunosuppressed transplant patients, in rheumatoid arthritis, and in B cell malignancies. Recently, Shuda et al. reported that a virus called Merkel cell polyomavirus (MCPyV) was integrated into the genome of 80% of human Merkel cell carcinomas analyzed by them. [5]
The five most common clinical features of markel cell carcinoma are summarized by the acronym, AEIOU: Asymptomatic/lack of tenderness, Expanding rapidly (≤3 months), Immunosuppression, Older than age 50, and location on a UV-exposed site. [6] It classically presents as a skin-colored, red, or violaceous, firm, and non-tender papule or nodule with a smooth and shiny surface. Reported atypical presentations include subcutaneous lesions, crusted erosion on the nose, chalazion-like palpebral lesion, conjunctival multinodular mass, granulation tissue-like lesions, plaque-like lesions and pedunculated lesions with a telangiectatic surface. Ulceration is seldom observed and found only in very advanced lesions. [7]
Histologically, the tumor mass is composed of uniformly small cells with scant cytoplasm and vesicular nucleus. Based on the size of the cells and their arrangement in sheets, nests, or trabeculae, three histologic patterns may be distinguished: trabecular or classical, formed of small cells; intermediate cell type, with large solid nests of cells that are not small in size; and small cell type, with a pattern of diffuse infiltrating sheets of small cells. Moulding of cells, which has been described as a ball-in-mitt arrangement, is noted frequently. Merkel cell carcinoma demonstrates expression of CK20 with characteristic dot-like paranuclear reactivity. CD56, neuron-specific enolase, and Bcl-2 (B cell lymphoma-2) are detected in 100% of cases; only 10% of cases are positive for CK7. The neuroendocrine differentiation of Merkel cell carcinoma is confirmed by positive staining for synaptophysin and chromogranin A. Metastatic small cell carcinoma may be excluded by absent expression of thyroid transcription factor-1, and lymphoma by the absence of CD45/ LCA (Leukocyte common antigen), CD20, and CD3. [8]
Our patient did not manifest any of the classical AEIOU features of Merkel cell carcinoma. Instead, there were many unusual features: a relatively young age of 40 years, the presence of pruritic lesions, slow progression over 1 year, involvement of a non-sun-exposed site, absence of any evidence of immunosuppression which is expected in early-onset Merkel cell carcinoma and the uncommon finding of CK7 positivity on biopsy. She presented with ulcerated plaques suggestive of an aggressive course, but there was no regional lymphadenopathy or evidence of distant metastasis.
Merkel cell carcinoma is known for its high rate of recurrence and metastasis. Despite its high degree of malignancy, spontaneous regression has been well documented. [9] Spontaneous regression appears to be more common in women, in the nodal site, when the primary site is the head or neck, and after biopsy or fine needle aspiration. Generally, both primary and metastatic merkel cell carcinoma that have regressed have been found to be infiltrated by immune cells, including T cells and macrophages. However, Mott et al. have reported a poor outcome with heavy lymphocytic infiltration. Individuals with Merkel cell polyoma virus positivity are thought to have a more favorable outcome, possibly due to the immune response triggered by the virus. [10]
Our patient showed complete regression of lesions within 1 month of biopsy. The presence of Merkel cell polyoma virus was not assessed in our case. The procedure of skin biopsy might have stimulated the immune system toward tumor destruction. Richetta et al., in their review of total spontaneous regression of advanced cases have mentioned female sex and biopsy as factors favouring tumor regression, both of which may have played a role in our patient′s outcome. [11] We were unable to find any previous reports of the association of CK7 positivity and spontaneous regression of Merkel cell carcinoma. The influence of CK7 positivity on the natural course of Merkel cell carcinoma is not yet known.
| 1. |
Calonje E. Tumours of the skin appendages. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8 th ed. Oxford: Blackwell Publishing; 2010. p. 50.42-3.
th ed. Oxford: Blackwell Publishing; 2010. p. 50.42-3.'>[Google Scholar]
|
| 2. |
Moll I, Roessler M, Brandner JM, Eispert AC, Houdek P, Moll R. Human Merkel cells-aspects of cell biology, distribution and functions. Eur J Cell Biol 2005;84:259-71.
[Google Scholar]
|
| 3. |
Toker C. Trabecular cell carcinoma of the skin. Arch Dermatol 1972;105:107-10.
[Google Scholar]
|
| 4. |
De Wolff-Peeters C, Marien K, Mebis J, Desmet V. A cutaneous APU Doma or Merkel cell tumor? A morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer 1980;46:1810-6.
[Google Scholar]
|
| 5. |
Majewska H, Biernat W. Merkel cell carcinoma. Pathological and molecular aspects of diagnosis and clinical features. Pol J Pathol 2010;61:117-23.
[Google Scholar]
|
| 6. |
Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The "AEIOU" features. J Am Acad Dermatol 2008;58:375-81.
[Google Scholar]
|
| 7. |
Errichetti E, Piccirillo A, Ricciuti F, Ricciuti F. Pedunculated and telangiectatic merkel cell carcinoma: An unusual clinical presentation. Indian J Dermatol 2013;58:243.
[Google Scholar]
|
| 8. |
Smith PD, Patterson JW. Merkel cell carcinoma (neuroendocrine carcinoma of the skin). Am J Clin Pathol 2001;115:S68-78.
[Google Scholar]
|
| 9. |
Kubo H, Matsushita S, Fukushige T, Kanzaki T, Kanekura T. Spontaneous regression of recurrent and metastatic Merkel cell carcinoma. J Dermatol 2007;34:773-7.
[Google Scholar]
|
| 10. |
Triozzi PL, Fernandez AP. The role of the immune response in merkel cell carcinoma. Cancers 2013;5:234-54.
[Google Scholar]
|
| 11. |
Richetta AG, Mancini M, Torroni A, Lore B, Iannetti G, Sardella B, et al. Total spontaneous regression of advanced merkel cell carcinoma after biopsy: Review and a new case. Dermatol Surg 2008;34:815-22.
[Google Scholar]
|
Fulltext Views
2,350
PDF downloads
1,472





