Translate this page into:
Spontaneous regression of primary cutaneous diffuse large B-cell lymphoma, leg type after biopsy
Corresponding author: Prof. Lin Wang, Department of Dermatology & Venereology, West China Hospital, Sichuan University, Chengdu, Sichuan, China. lkzwl@126.com
-
Received: ,
Accepted: ,
How to cite this article: Li F, Wang L. Spontaneous regression of primary cutaneous diffuse large B-cell lymphoma, leg type after biopsy. Indian J Dermatol Venereol Leprol 2023;89:110-113.
Sir,
Primary cutaneous diffuse large B-cell lymphoma, leg type usually shows an intermediate-to-aggressive behaviour. We report a case of this disease that spontaneously regressed after biopsy without any anti-neoplastic treatment, which is extremely rare.
A previously healthy 62-year-old man was referred to our department in April 2016, with complaints of five painless nodules on his left leg, since the last one month. These lesions initially appeared as small red papules, which rapidly increased in size and depth within a few days. The patient had no fever, pain, pruritus or any other symptoms. He underwent a biopsy in a local hospital two weeks ago, but no conclusive diagnosis was established. He was treated with topical corticosteroids with no improvement.
Physical examination revealed five erythematous, indurated nodules measuring 1–4 cm in diameter aggregated on the posteromedial side of his left leg [Figure 1a], with no increase in local temperature. No lymphadenopathy was present. The results of the routine laboratory tests including serum lactate dehydrogenase, coagulation profile, autoantibodies and protein electrophoresis were all unremarkable, as were the serologic tests for HIV, Treponema pallidum, hepatitis B virus and hepatitis C virus and serology and antigen detection for Epstein-Barr virus.
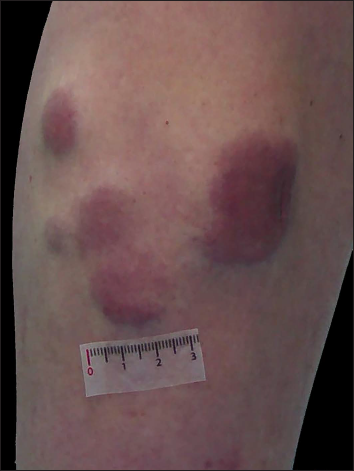
- Five erythematous, indurated and infiltrated nodules and tumours on the left leg
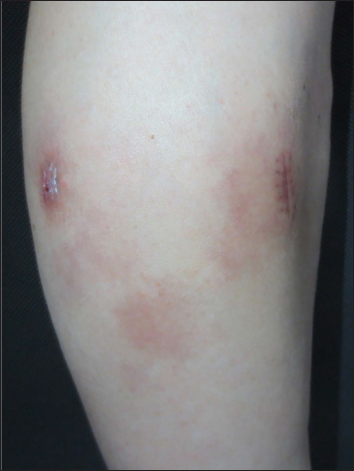
- Most of the original lesions regressed spontaneously after 3 months, leaving varying degrees of pigmentation
Histopathological examination of a second biopsy revealed dense infiltrate of large lymphoid cells throughout the dermis and subcutis [Figure 2a], most of which showed features of centroblasts and immunoblasts with prominent nuclei and mitotic activity [Figure 2b], along with a scattering of few small lymphocytes in between. An obvious non-involved band was seen between the epidermis and dermal infiltrate. Immunohistochemical analysis revealed positivity for cluster of differentiation (CD)20, CD79a, B-cell leukemia/lymphoma (Bcl)-6 and paired box gene (PAX)-5, partial positivity for Bcl-2 and multiple myeloma protein (Mum)-1 [Figures 3a–e], and negativity for CD10, CD3, CD30 and CD138. Ki-67 (also called mind bomb 1 protein, MIB-1) proliferation rate was around 60% [Figure 3f]. Epstein-Barr-encoded RNA in situ hybridisation was negative. Polymerase chain reaction detected clonal rearrangement of both IgH and IgK. The bone marrow examination including smear, flow cytometry and biopsy showed no evidence of malignancy. No other obvious anomaly was found in a positron emission tomography either. The diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type was made. The patient refused to undergo any treatment because his skin lesions began to flatten spontaneously after the second biopsy. At 3 months from the first visit, the tumours had undergone almost complete resolution, leaving slight post inflammatory hyperpigmentation [Figure 1b]. During the following 60 months, the patient still remained disease-free.
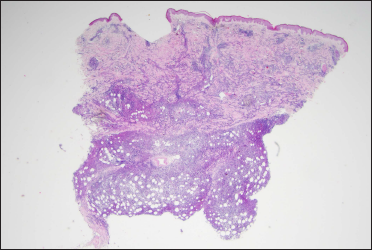
- A diffuse infiltration distributed throughout the dermis and subcutis (haeamatoxylin and eosin stain, ×12.5)
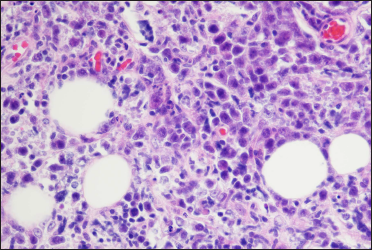
- A diffuse infiltration of large cells with prominent nuclei distributed throughout the dermis and subcutis mainly including centroblasts and immunoblasts (haematoxylin and eosin stain, ×400)
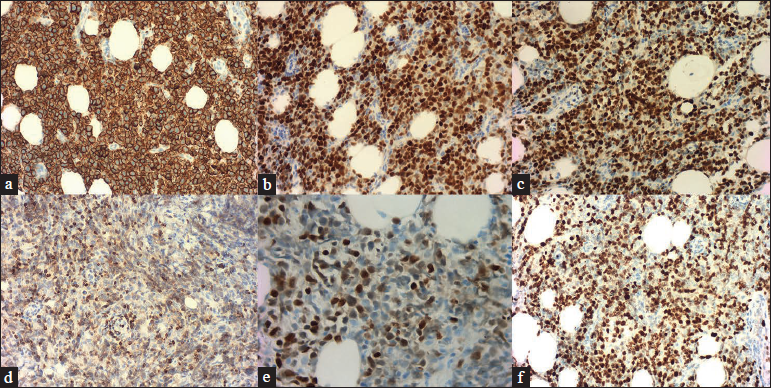
- The neoplastic cells were positive for (a) CD20 (x200), (b) Bcl-6 (×200) and (c) PAX-5 (×200). The neoplastic cells were partial positive for (d) Bcl-2 (×200) and (e) Mum-1 (×400). (f) The Ki-67 proliferation rate was around 60% (×200).
Primary cutaneous diffuse large B-cell lymphoma, leg type is an intermediate-to-aggressive subtype of B-cell lymphoma that originates in the skin without extracutaneous dissemination at diagnosis. It is more common in the elderly (median age ≈76 years) and females (female:male≈ 2:1),1 and the typical manifestation is erythematous to violaceous infiltrated nodules, tumours or plaques usually limited to legs. Primary cutaneous diffuse large B-cell lymphoma, leg type needs to be distinguished from other subtypes of primary cutaneous B-cell lymphoma, since it has a less favourable prognosis, with 5-year survival rate of around 50% only,1 and the recommended treatment is multidrug chemotherapy.
Spontaneous regression has been described previously for many tumours as a partial or complete disappearance of the tumour without any therapeutic intervention. For example, up to 50% of primary cutaneous melanoma has been reported to undergo spontaneous regression, but there is still a controversy over its effect on the prognosis of melanoma. Comparatively, spontaneous regression is rare in intermediate or aggressive non-Hodgkin’s lymphomas. However, in recent years, several cases of diffuse large B-cell lymphoma regressing spontaneously have been reported, the majority of which were the extra-nodal ones, mainly including head, breast, stomach and skin. In total, we could find only five reported cases2-6 of spontaneous regression in primary cutaneous diffuse large B-cell lymphoma, leg type [Table 1]. Only one other case presented with multiple lesions similar to our case.
| Case | Age/sex | Location | Skin lesions | Symptoms | Evolution of the lesions | Inflammatory cells in repeat biopsy during tumour regression | Complementary therapy | Outcome | Relapse |
|---|---|---|---|---|---|---|---|---|---|
| 12 | 82/F | Right leg | Multiple nodules and tumours | No | Almost disappeared after 4 weeks | Diffuse mature T cells (CD3+) | None | CR for 4 months until died of a cerebrovascular accident | No |
| 23 | 72/F | Upper left arm | Solitary tumour | No | Apparently shrank after the biopsy | Many small lymphocytes (positive for CD3, CD4, CD8, perforin, granzyme B and TIA1) | Surgery | CR for 21 months after surgery | No |
| 34 | 83/F | Back of the right leg | 2 erythematous nodules | Local pain | Regressed at 3 months after biopsy | Small-sized T lymphocytes (positivity for CD2, CD3, CD4, and predominantly CD8) | Radiotherapy | CR for 1 year after radiotherapy | No |
| 45 | 66/M | Lower left leg | Solitary tumour | No | Regressed after 2 months | Significant CD3+ T cells | Radiotherapy | ND | ND |
| 56 | 79/M | Anterior left leg | An erythematous reticulated plaque | No | Resolved 1 month later | Predominant CD8+ T-cells | None | CR for more than 1 year but ultimately relapsed | Yes |
| 6* | 62/M | Posteromedial left leg | 5 erythematous nodules | No | Regressed after 3 months | ND | None | CR for 60 months | No |
*The last one is our case. F: female, M: male, CR: complete remission, TIA1: cytotoxic granule associated RNA binding protein, ND: not described
Although the definite mechanism contributing to the spontaneous regression of lymphomas remains unclear, several hypotheses have been proposed in the literature, mainly involving modulation of the host immune system, complex interactions of tumour microenvironment and apoptosis.7 As suggested by most scholars, the activation and enhancement of the host immune response especially T cell mediated responses, are the most relevant. In all these reported cases of the biopsy specimens taken from the area of tumour regression revealed significant infiltration with small T lymphocytes,2-6 which were predominantly CD8+ T lymphocytes in some cases.4,6 Unfortunately, we failed to obtain a specimen from our patient during the regression stage of the tumour.
An infection or a physical disruption like trauma or surgical intervention can activate the anti-tumour immunity of the host. The underlying immune process is complicated, and involves sudden recognition of the neoplastic cells by the immune system, the non-specific anti-tumour effect of natural killer cells, and specific T cells cross-reacting with tumour antigens.7 As our patient did not have any evidence of immunosuppression or infection, it is possible that the injury and secondary inflammation of the local tissue induced by biopsy might have contributed to the anti-tumour process.
With increasing reports of spontaneous regression possibly mediated by host immune response, immunotherapy may gain more favour as a therapeutic option to treat primary cutaneous diffuse large B-cell lymphoma, leg type. One must also watch for a reduction in lesion size after biopsy and it may even herald the onset of a complete resolution. However, the case of primary cutaneous diffuse large B-cell lymphoma, leg type reported by Graham et al.5 ultimately suffered a relapse in the absence of systemic treatment after a >1 year period of regression, though the second biopsy of the regression area showed no evidence of atypical lymphoid cells, suggesting that further studies are needed to define the characteristics and prognostic factors for recurrences.
For the patients like our case, watchful waiting or an additional biopsy may be considered before going ahead with more toxic multi-drug chemotherapy. Meanwhile, closer medical observation and more robust data collection are needed to verify these hypotheses.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
List of abbreviations
CD: Cluster of Differentiation
Bcl: B-cell leukemia/lymphoma
Mum-1: Multiple myeloma protein-1
Ki-67: marker of proliferation Ki-67 protein, also called mind bomb 1 protein (MIB-1).
PAX-5: paired box gene 5
References
- Primary cutaneous B-cell lymphomas: Part I. Clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous regression of primary diffuse large B-cell lymphoma, leg type. Actas Dermosifiliogr. 2014;105:78-83.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous regression of a primary cutaneous diffuse large B-cell lymphoma, leg type. J Dermatol. 2017;44:608-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary cutaneous diffuse large B-cell lymphoma, leg type, with spontaneous regression after biopsy. Am J Dermatopathol. 2017;39:785-7.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous regression of primary cutaneous diffuse large B-cell lymphoma, leg type with significant T-cell immune response. JAAD Case Rep. 2018;4:305-9.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous regression of primary cutaneous diffuse large B-cell lymphoma, leg type. Acta Derm Venereol. 2018;98:608-9.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous regression of malignant tumors: Importance of the immune system and other factors (Review) Oncol Lett. 2010;1:941-5.
- [CrossRef] [PubMed] [Google Scholar]





