Translate this page into:
Sturge–Weber syndrome coexisting with multiple vertebral vascular malformations and hemivertebra with scoliosis and upper limb and ear hypertrophy
Correspondence Address:
Angoori Gnaneshwar Rao
Department of Dermatology, SVS Medical College, Mahbubnagar, Telangana
India
| How to cite this article: Rao AG, Reddy V S, Parimala M D, Tejal M, Fathima K, Preeti S, Jhawar J, Dharani V, Shruthi T. Sturge–Weber syndrome coexisting with multiple vertebral vascular malformations and hemivertebra with scoliosis and upper limb and ear hypertrophy. Indian J Dermatol Venereol Leprol 2020;86:187-190 |
Sir,
Sturge–Weber syndrome is a neurocutaneous disorder characterized by leptomeningeal vascular malformations and nevus flammeus or portwine stain, involving the ophthalmic (V1) and maxillary (V2) distribution of the trigeminal nerve. It is usually associated with ocular manifestations, such as, buphthalmos and glaucoma.
An 18-year-old male presented to the dermatology department of SVS Medical College and Hospital with deep purple-colored patches involving the scalp, face, neck, chest and both upper limbs since birth and weakness of right upper limb for the last 2 years. He denied history of seizures, shortness of breath, visual disturbances, pain abdomen and hematuria. Examination revealed deep purple-colored patches over the right parietal, right periorbital region, right ear and face, right side of neck, front of chest and both upper limbs [Figure - 1]. There was hypertrophy of right upper limb, with a girth difference of 1.5 cm between the right and the left side [Figure - 2]. Multiple deep purple-colored nodules were surmounted sparsely on the port-wine stain, ranging from 0.5 to 2 cm in size. These were soft, compressible, non-pulsatile and non-tender. He was provisionally diagnosed as Sturge Weber Syndrome Type 1. However, overlap of Sturge Weber Syndrome with Klippel–Trenauny syndrome and Parkes–Weber syndrome were considered in the differential diagnosis. Absence of varicose veins in the index case was not supportive of the diagnosis of the overlap syndrome. Parkes–Weber syndrome could not be entertained, as there was no clinical evidence of arterio-venous malformations, such as increased warmth of the limb and bone hypertrophy. Routine laboratory and hematological investigations were non-contributory. Ophthalmic examination, including fundoscopy revealed normal study. MRI with contrast of the spine showed evidence of vascular malformations in the vertebral bodies. Large vascular malformations were noted in the 6th, 7th, 9th and 10th thoracic vertebrae and in the 2nd lumbar vertebra; while small vascular malformations were found in the 3rd, 8th and 11th thoracic vertebrae [Figure - 3] and in the 7th cervical hemivertebra [Figure - 4]. There was no extension of the port-wine stain over the vertebrae. MRI brain showed extradural vascular malformations in the right frontal, parietal and occipital region [Figure - 5]. MRI of long bones, ultrasonography of abdomen, 2D echocardiography and chest skiagram revealed normal study. Histopathological examination of biopsy from the port-wine stain and nodule showed scattered dilated blood vessels with blood elements and endothelial proliferation in the dermis and fibro-collagenous tissue with adnexal structures in the intervening stroma, suggestive of cavernous hemangioma [Figure - 6] and [Figure - 7]. Diagnosis of Sturge-Weber syndrome type I with multiple vertebral vascular malformations, C7 hemivertebra with scoliosis, and unilateral upper limb and ear hypertrophy was established. He was kept under neurosurgical observation as leptomeningeal and vertebral vascular malformations were asymptomatic and pulsed dye laser therapy was advised for discernible port-wine stain.
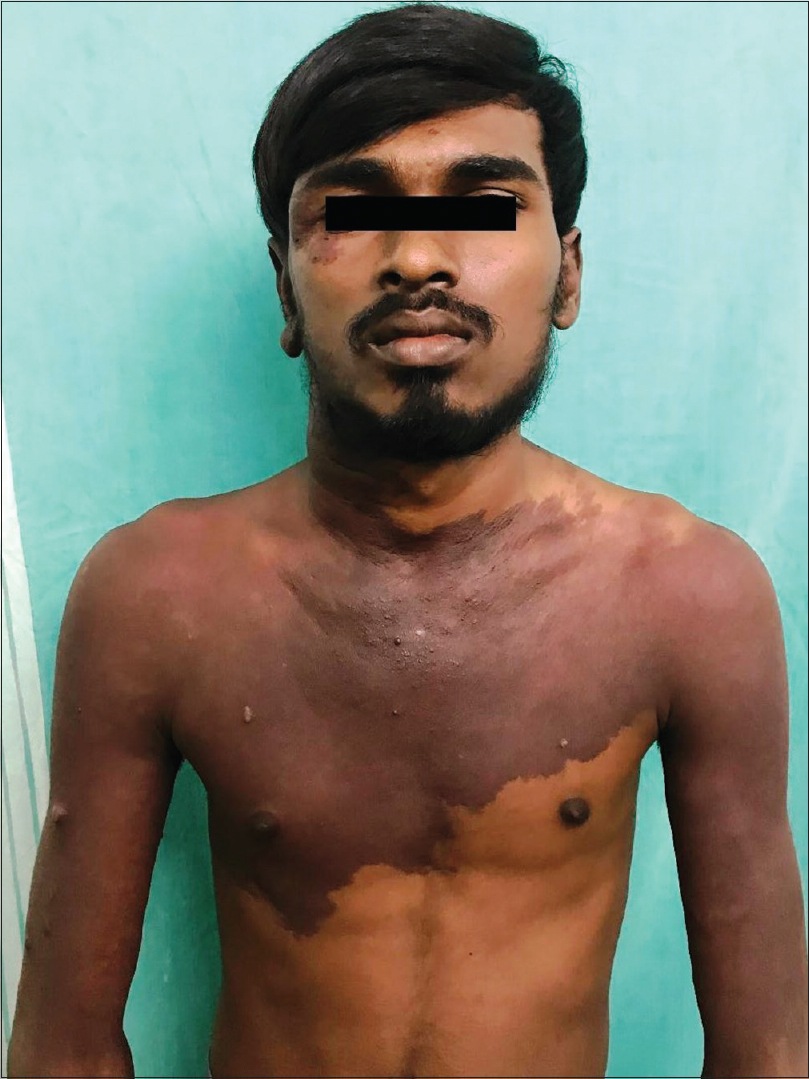 |
| Figure 1: Port-wine stain involving face, neck and front of chest with multiple deep purple nodules and hypertrophy of right ear is notable |
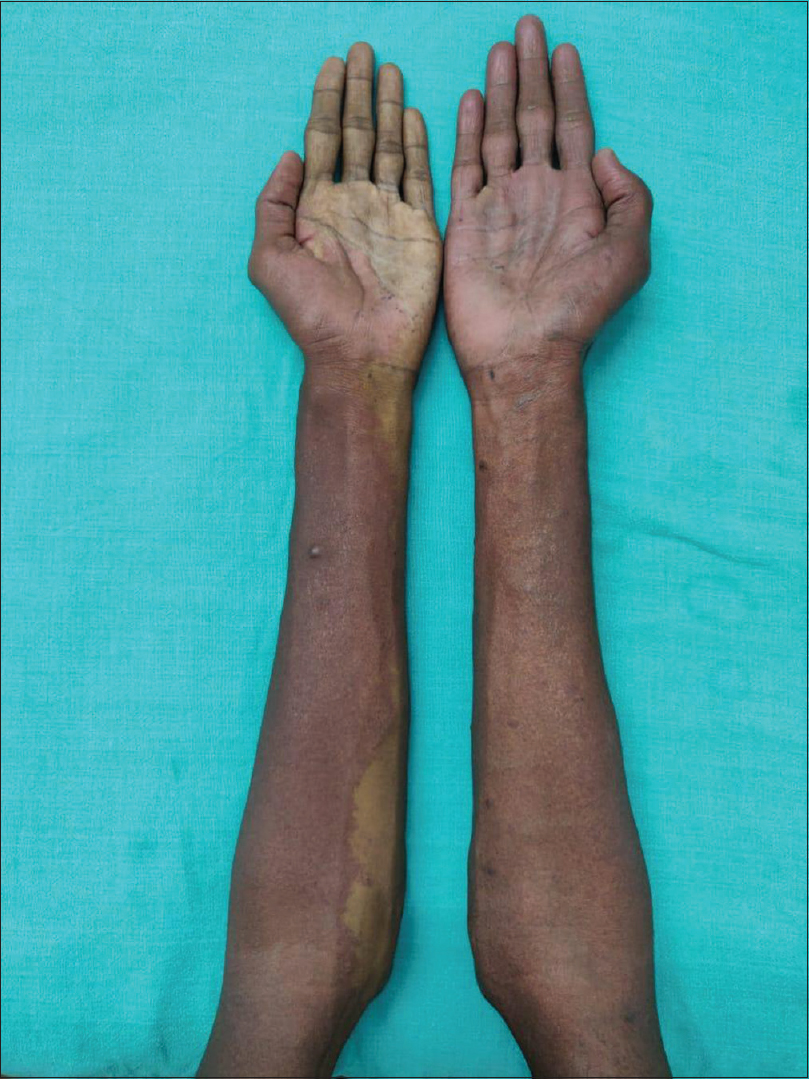 |
| Figure 2: Hypertrophy of right upper limb with port-wine stain involving both forearms |
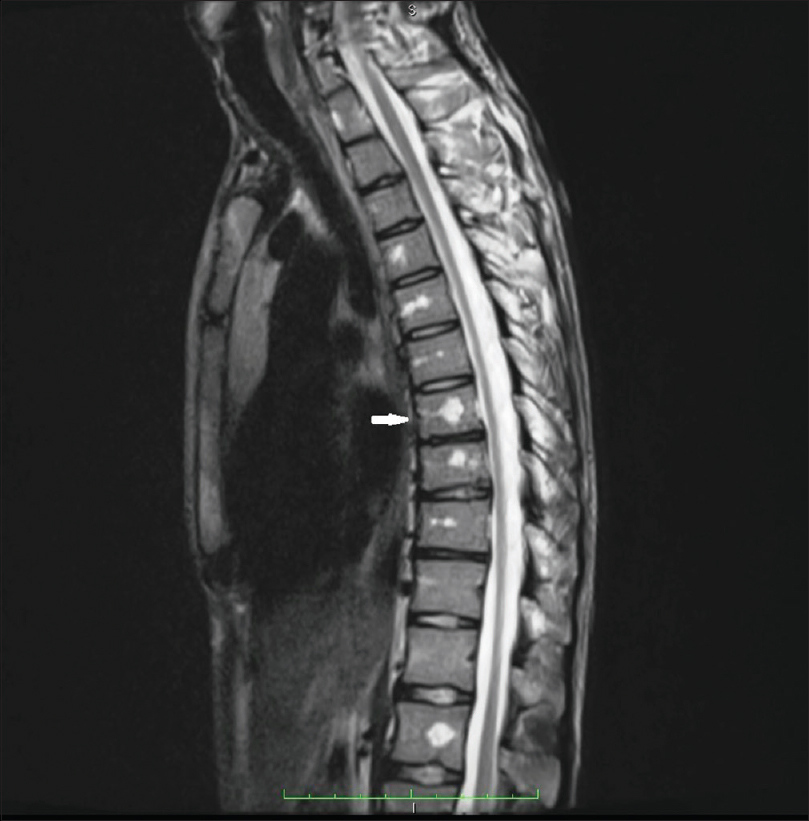 |
| Figure 3: Magnetic resonance imaging with contrast of spine; large vascular malformation in T6, T7, T9, T10, L2 and small vascular malformations in T3, T8, T11. Arrow shows large vascular formation |
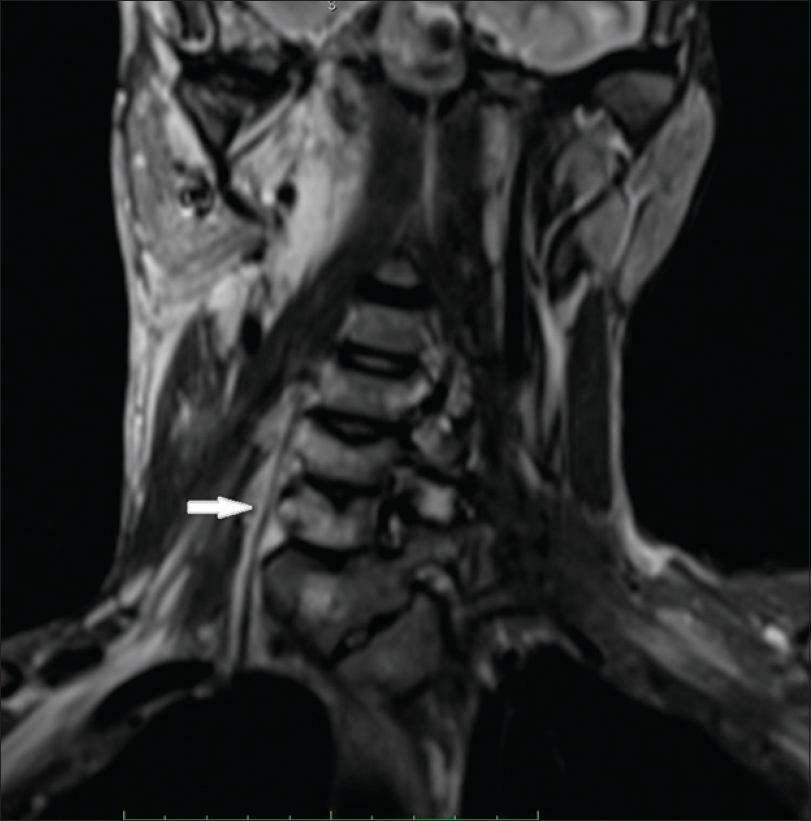 |
| Figure 4: Magnetic resonance imaging with contrast spine; white arrow shows C7 hemivertebra |
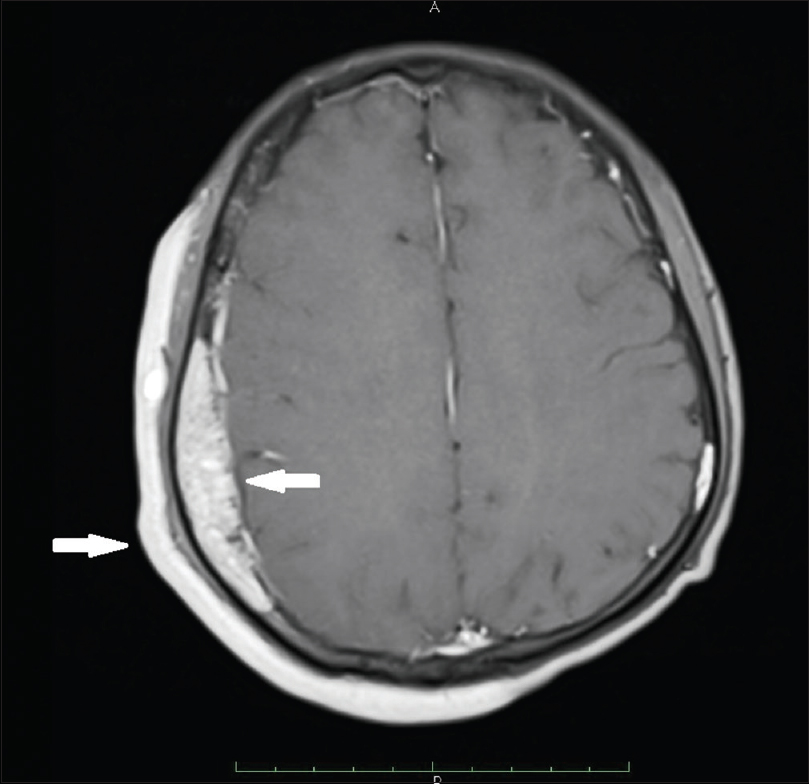 |
| Figure 5: Magnetic resonance imaging brain; extradural vascular malformations in right frontal, parietal (central arrow) and occipital region. Outer arrow shows port-wine stain |
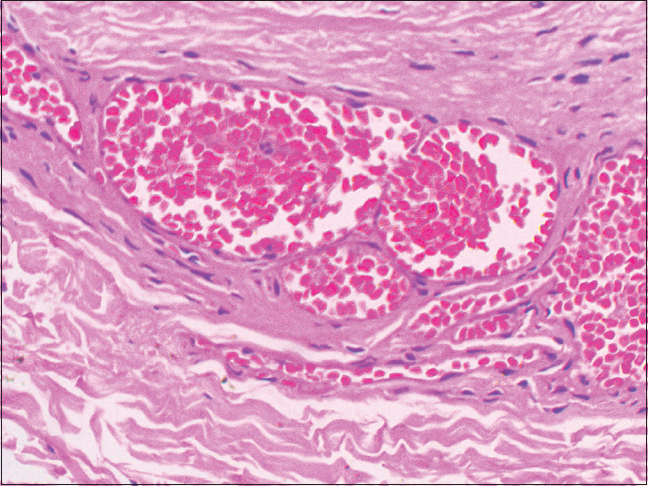 |
| Figure 6: Histopathology of port-wine stain; scattered dilated blood vessels with blood elements and endothelial proliferation in the dermis and the intervening stroma (H and E, ×400) |
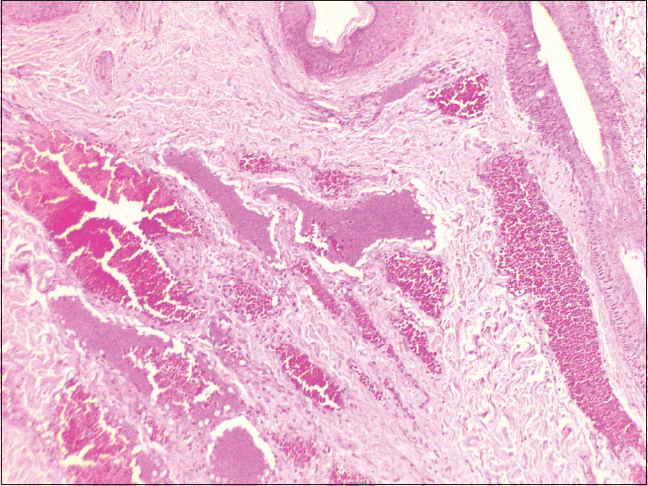 |
| Figure 7: Histopathology of deep purple nodule; adnexal structures with intervening stroma showing dilated cavernous blood vessels filled with blood elements (H and E, ×100) |
Sturge-Weber syndrome is caused by a somatic mutation in the GNAQ gene located at 9q21.2, which is the long (q) arm of chromosome 9 at position 21.2. The enrichment of endothelial cells noted in the skin capillary malformations associated with this syndrome, is known to be induced by GNAQ mutation.[1]
The most common manifestation of this syndrome is a port-wine stain on the face, reported in 87–90%; typically located near or around an eye or on the forehead, and varies in size and color. It generally occurs on the right side; however, bilateral involvement has been reported in 31% of cases.[2] Proliferation of blood vessels in the brain results in abnormal brain function with subsequent development of seizures. Most patients suffer from seizures before one year of age; seen in roughly 75% of patients with unilateral brain involvement, and 95% with bilateral brain involvement. Moreover, 8–20% of patients with port-wine stain are known to suffer from neurological symptoms and the risk is about 35% when extensive bilateral facial port-wine stains are present.[3] Interestingly, seizures were not encountered by the index case despite giant port-wine stains and large extradural vascular malformations.
The coexistence of multiple vascular malformations in the vertebral bodies (T3, T6, T7, T8, T9, T10, T11 and L2) with Sturge-Weber syndrome, is rare. Nonetheless, there have been reports of hemangiomas involving the vertebrae, unassociated with this syndrome in the literature. Most of them are symptomatic and present with symptoms of spinal cord or nerve root compression.[4] Similarly, Fox and Onofrio also reported compression symptoms in 35 (61%) patients in a study of 59 cases of vertebral hemangiomas.[5] Hemangiomas involving thoracic vertebrae are more likely to cause compression than those of lumbar vertebrae.[6] It is interesting to note, that our patient is asymptomatic even though vascular malformations involved multiple thoracic and lumbar vertebrae. However, long-term follow-up may divulge the effects of vascular malformations on these vertebrae. Furthermore, the association of Sturge-Weber syndrome with C7 hemivertebra is also rare. The scoliosis and wasting of palmar muscles on right side in the index case, may be attributable to the C7 hemivertebrae. Interestingly, McMaster and David studied 104 patients, with total 154 hemivertebrae which produced scoliosis. They inferred that the development of scoliosis depends on several factors that included type of hemivertebra, its site, number, relationship to each other, and age of the patient.[7]
Limb hypertrophy is the usual accompaniment of Klippel–Trenauney syndrome. Occurrence of hypertrophy of the right upper limb and right ear in the index case is notable and rare.
Approximately, 50% of these patients show ocular changes, usually on the same side as port-wine stain, involving the eyelid, anterior chamber, cornea, choroid and retina.[8] In addition, 30–70% patients with this syndrome develop glaucoma. Absence of eye involvement in our patient is noteworthy.
In view of the multisystem involvement, multidisciplinary approach is imperative in the management of these cases. Radiation therapy and embolization are effective in the management of symptomatic patients with vertebral vascular malformations. As port-wine stain involved large areas of the body, he was advised multiple sessions of pulsed dye laser therapy for discernible areas.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Huang L, Couto JA, Pinto A, Alexandrescu S, Madsen JR, Greene AK, et al. Somatic gnaq mutation is enriched in brain endothelial cells in sturge-weber syndrome. Pediatr Neurol 2017;67:59-63.
[Google Scholar]
|
| 2. |
Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, Viaño J. Sturge-weber syndrome: Study of 55 patients. Can J Neurol Sci 2008;35:301-7.
[Google Scholar]
|
| 3. |
Tallman B, Tan OT, Morelli JG, Piepenbrink J, Stafford TJ, Trainor S, et al. Location of port-wine stains and the likelihood of ophthalmic and/or central nervous system complications. Pediatrics 1991;87:323-7.
[Google Scholar]
|
| 4. |
Alfawareh M, Alotaibi T, Labeeb A, Audat Z. A Symptomatic case of thoracic vertebral hemangioma causing lower limb spastic paresis. Am J Case Rep 2016;17:805-9.
[Google Scholar]
|
| 5. |
Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg 1993;78:36-45.
[Google Scholar]
|
| 6. |
Vinay S, Khan SK, Braybrooke JR. Lumbar vertebral haemangioma causing pathological fracture, epidural haemorrhage, and cord compression: A case report and review of literature. J Spinal Cord Med 2011;34:335-9.
[Google Scholar]
|
| 7. |
McMaster MJ, David CV. Hemivertebra as a cause of scoliosis. A study of 104 patients. J Bone Joint Surg Br 1986;68:588-95.
[Google Scholar]
|
| 8. |
Comi AM, Mehta P, Hatfield LA, Dowling MM. Sturge-Weber syndrome associated with other abnormalities: A medical record and literature review. Arch Neurol 2005;62:1924-7.
[Google Scholar]
|
Fulltext Views
5,973
PDF downloads
3,037





